Lipophilic Dye Kits for Cell Tracking
1 Adapted from Table 1 of Tario Jr. et al. (2011); Reprinted with permission from Humana Press. First published in Methods in Mol. Biology, 699, 119 (2011).
Abbreviations: APC = allophycocyanin; 7-AAD= 7-aminoactinomycin D; CFSE = carboxyfluorescein succinimide ester; Cy3 = cyanine 3; Cy5 = cyanine 5; Cy7 = cyanine 7; FPs = fluorescent proteins; PE = phycoerythrin; PI = propidium iodide; PerCP = peridinin chlorophyll lprotein; TR = Texas Red*
* CellVue is a trademark of PTI Research, Inc. CY is a trademark of Cytiva. Texas Red and TO-PRO-3 are trademarks of Molecular Probes. DRAQ5 is a trademark of Biostatus Ltd.
1 See Refs. 1-4 and11 for further details. Originally developed by Paul Karl Horan and colleagues at Zynaxis Cell Science in the late 1980’s, PKH26 been distributed by us since 1993 and PKH67 since 1997. CellVue Claret, a far red analog developed by PTI Research, Inc. was added to our family of cell tracking kits in 2008.
2 Spectral compatibilities and incompatibilities are representative, not exhaustive, and assume that laser excitation spots are spatially separated, not coincident. Relative signal from different fluorescent probes will vary depending on the biological system of interest. Each user must run the necessary controls to verify compatibility in their own system.
3 Shcherbo D, Merzylak EM, Chepurnykh TV et al. Bright Far-red Fluorescent Protein for Whole-body Imaging. Nature Meth. 4:741-746 (2007).
4 <0.3% @ 24h when FBS or other protein is used as “stop” reagent in staining protocol (see text).
5 For partial bibliography of PKH dye applications (1988 – 2006), see Tables 1 and 2.
6 See Refs. 3, 8, 9, 12 and 20.
7 See Refs. 3 and 11.
8 GL kits contain 0.5 mL dye + 6x10mL Diluent C (recommended for general membrane labeling of cells for large or in vivo studies).
9 MIDI kits contain 2x0.1 mL dye + 6x10mL Diluent C (recommended for general membrane labeling of cells for in vitro proliferation or cytotoxicity studies).
10 MINI kits contain 0.1 mL dye + 1x10mL Diluent C (recommended for general membrane labeling of cells for small or preliminary in vitro studies).
11 PCL kits contain 0.5 mL dye + 6x10mL Diluent B (recommended for selective labeling of phagocytes in the presence of non-phagocytes for in vitro or in vivo studies).
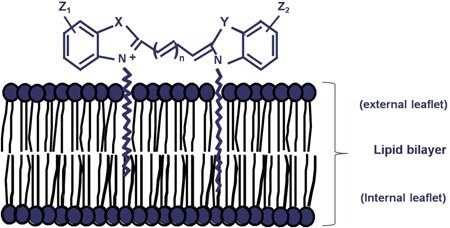
Figure S1.Staining mechanism
The PKH and CellVue dyes are lipid-like molecules with fluorescent "head groups" and long aliphatic "tails" (not shown to scale). In salt-containing buffers or media they rapidly form micelles or aggregates with poor cell labeling efficiency for non-phagocytic cells. Diluent C (the iso-osmotic, salt- and solvent-free staining vehicle provided in our general membrane labeling kits) maximizes dye solubility and facilitates near-instantaneous partitioning into the lipid bilayer. Strong non-covalent interactions with surrounding lipid tails provide long term dye retention and stable fluorescence intensities in non-dividing cells.
Reprinted with permission from John Wiley & Sons, Inc. First published in Cytometry, 73A. 1019 (2008).
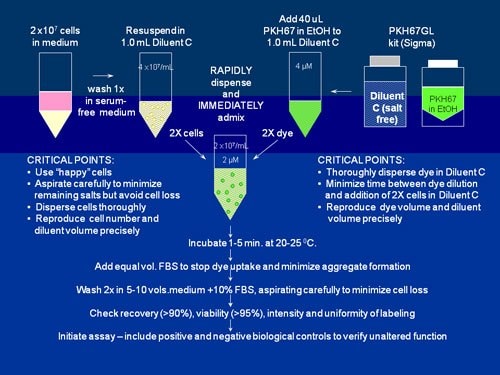
Figure S2.General membrane labeling protocol for PKH26, PKH67 and CellVue Claret
When using the salt-free Diluent C vehicle, provided with our cell linker kits to maximize staining efficiency and homogeneity, partitioning of these highly lipophilic dyes into cell membranes occurs almost instantly upon mixing with cells. As illustrated in this schematic for labeling with PKH67, bright, uniform and reproducible staining is most readily obtained by: 1) minimizing the amount of salts present in the staining step and 2) insuring rapid homogeneous dispersal of cells in dye. (For detailed methods and staining protocols, see Refs. 2 and 3 and links to individual product bulletins included in Table S2.)
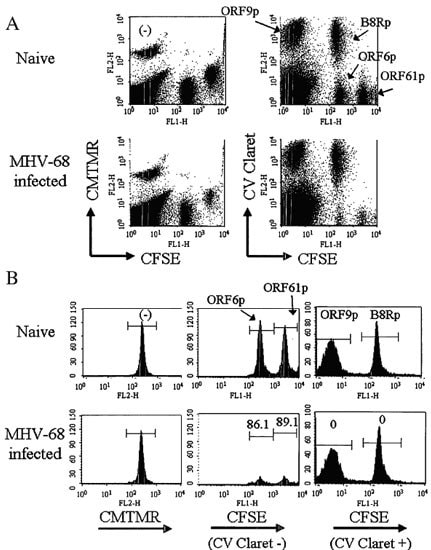
Figure S3.Color coding of target populations allows simultaneous comparison of in vivo CTL cytotoxicity against multiple epitopes in a single mouse
Unpulsed splenocytes or splenocytes pulsed with 1 of 4 immunogenic MHV-68 peptides for 1 h @ 37 °C. were labeled with distinct 1 or 2-color combinations of 3 different cell tracking dyes. Single staining with CMTMR identified unpulsed splenocytes, whereas peptide-pulsed splenocytes were labeled with combinations of CellVue Claret and CFSE. The 5 labeled target populations were then admixed in equal numbers, injected into naïve mice or mice that had been infected with MHV-68 virus 10 days prior (3 per group), and spleens were harvested 4 hours post-injection. After red cell lysis, the frequency of viable targets in each population was assessed by flow cytometry, using light scatter gating to exclude debris and aggregates and 7-AAD uptake to exclude non-viable cells. [NOTE: Although the peptides shown here were candidate epitopes for an antiviral vaccine, a similar strategy could be applied to identification of efficacious anti-tumor vaccine components.]
A. Representative two color plots showing tracking dye combinations used to identify target populations pulsed with each of the peptides (CMTMR only: No peptide; CFSEhi CellVue Claretneg: ORF61p; CFSElo CellVue Claretneg: ORF6p; CFSEneg CellVue Claretpos: ORF9p; CFSEhi CellVue Claretpos: B8Rp).
B. Representative single color histograms used to determine epitope specificity of killing. Left panels: CMTMR gated. Center panels: CFSE distribution of CellVue Claretneg targets. Right panels: CFSE distribution of CellVue Claretpos targets). Values on lower panels indicate percent specific lysis, calculated based on the change in frequency of viable targets seen in infected vs. naïve animals (see Ref. 9 for details).
Reprinted with permission from Informa Healthcare. First published in Immunol. Invest.36, 829 (2007).
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?