A Serum-Free and Feeder-Free Defined Medium That Enables Weekend-Free Culture of Undifferentiated Human ES/iPS Cells
Section Overview
Introduction to Stem Cell Culture in PluriSTEM™ Media
Current serum-free media formulations used to culture human pluripotent stem cells require daily media replenishment and at least one media exchange on the weekend. The costs in media, reagents, and human resources become prohibitively expensive as increasing numbers of core labs and consortia are focused on generating disease models using human iPS cells. PluriSTEM™ Human ES/iPS Cell Media are small molecule-based media that enable weekend-free culture of human pluripotent stem cells and allow for media exchanges every other day without compromising the morphology or long-term functionality of pluripotent stem cells. Pluripotent cells maintained in this moderate feeding regimen exhibited robust cell health with minimal spontaneous differentiation, expressed high levels of pluripotency markers (Nanog, Oct-4, Sox-2, SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81), retained differentiation potential, and possessed a normal karyotype.
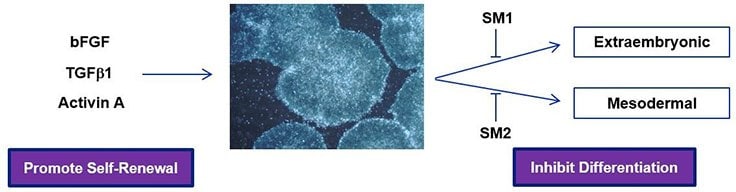
Figure 1.Human pluripotent stem cells grown in PluriSTEM™ media retain pluripotency and demonstrate robust growth rates via two mechanisms: 1) bFGF, TGFβ1 and Activin A promote stem cell self-renewal and 2) small molecule inhibitors suppress unwanted spontaneous extraembryonic and mesodermal differentiation pathways.
Methods for Culture of Stem Cells in PluriSTEM™ Media
Preparation of Coated Plates for Stem Cell Culture
Expansion of pluripotent human ES and iPS cells with PluriSTEM™ media requires cultureware that are coated with ECM Gel Matrix (CC131) or xeno-free substrates such as Vitronectin (CC130) or ECMatrix™-511 Laminin Substrates. Below are general guidelines for the coating of 6-well plates and culture flasks with ECM Gel Matrix:
- Thaw ECM Gel Matrix (CC131) on ice. Keep on ice and use pre-cooled medium and pipettes to avoid premature gelation of the ECM reagent. IMPORTANT: To prevent unintentional gelling, do not thaw ECM Gel Matrix at temperatures higher than 15 °C
- Dilute the ECM Gel Matrix 1:20 with cold DMEM/F12 (D6421). For example, to every 0.5 mL ECM Gel Matrix, add 9.5 mL cold DMEM/F12 medium for a total volume of 10 mL. Scale according to the volumes required.
- Cover the cultureware surface with the recommended volumes (for example: add 1.5 mL to each well of a 6-well plate). Swirl the culture plates to spread the ECM Gel Matrix evenly across the surface of the plate. Incubate at room temperature for at least 1 hour or 2–8 °C overnight. If not used immediately, store coated cultureware at 2-8 °C until ready to use. Note: If not used immediately, coated cultureware should be sealed with parafilm to prevent evaporation and can be stored at 2 – 8 °C for up to one week.
- Prior to seeding the cells, bring the plate back to room temperature, remove the coating solution and add an appropriate volume of PluriSTEM™ Human ES/iPS Medium (SCM130, SCM132). IMPORTANT: Do not allow the flask to dry out.
Preparation of Dispase II for Stem Cell Culture
Pluripotent human ES and iPS cells maintained in PluriSTEM™ media may be enzymatically passaged using Dispase II protease. PluriSTEM™ Dispase II Solution (SCM133) is a ready-to-use 1 mg/mL solution validated to work alongside PluriSTEM™ Human ES/iPS Media for the culture and passage of human embryonic and induced pluripotent stem cells.
Transition of Human ES/iPS Cells to PluriSTEM™ Media
(Applicable for both Feeder-Based and Feeder-Free Media Systems)
- One to two days prior to passaging, exchange the media with 3 mL PluriSTEM™ media (SCM130, SCM132) per well of a 6-well plate. Exchange with fresh PluriSTEM™ media the next day.
- On the day of passaging, acclimate ECM Gel Matrix (CC131) coated plate for 1 hour at room temperature. After 1 hour, remove the coating. Add 2 mL PluriSTEM™ media to each well. Set plate aside until cells are ready to be passaged.
- Aliquot sufficient PluriSTEM™ media, Dispase II (SCM133) and DMEM/F12 (D6421) to passage the cells. Warm reagents at room temperature (15–25 °C) for 5 – 10 minutes.
- Use a dissection microscope to visually inspect the plate containing human pluripotent cells to be passaged. Inspect the colonies for areas of spontaneous differentiation. Note: Areas of spontaneous differentiation are characterized as phase-bright, highly dense areas with irregular borders, non-uniform cell morphologies and cell types and are typically localized either in the center of the colonies or along the edges between colonies.
- Use a sterile p200 pipette tip attached to a p200 pipetman to scrape away areas of spontaneous differentiation. Be discriminating and scrape away any areas that harbor a hint of differentiation. Note: It is critical to start with high quality undifferentiated human ES and iPS culture. When maintained correctly, spontaneous differentiation should be <1 – 5% in PluriSTEM™ media culture.
- Aspirate the medium containing the scraped areas from the well. Rinse with 2mL per well of DMEM/F-12 medium or 1X PBS.
- Add 1 mL Dispase II per well of the 6-well plate containing pluripotent human ES or iPS cells to be passaged.
- Incubate at 37°C for 5-10 minutes. After incubation, visually inspect the colonies under a microscope. The edges of the colonies may appear slightly rounded up and folded back, but most of the colony should still be attached to the plate.
- Aspirate the Dispase II solution and gently rinse each well two times with 2 mL 1X PBS or DMEM/F12 medium to remove any residual Dispase II solution. Aspirate after each rinse.
- Add 1.5–2 mL PluriSTEM™ media to each well. Gently detach the colonies using a cell scraper.
- Use a 5 mL serological pipette to collect the cell aggregates to a 15 mL conical tube. Minimize pipetting up and down as this may break up the colonies to suboptimal small pieces. The process of transferring the cell aggregates to the 15 mL conical tube should be sufficient to disaggregate the colonies to optimal size.
- Rinse the wells with an additional 2 mL of PluriSTEM™ media per well to collect any remaining cell aggregates. Add the rinse to the 15 mL conical tube.
- Centrifuge the 15 mL conical tube containing the cell aggregates at 300 x g for 5 minutes at room temperature (15–25 °C).
- Aspirate the supernatant. Resuspend the cell aggregates in an appropriate volume of PluriSTEM™ media for passaging. Do not pipette the cell aggregates more than 1–2 times with a 5 mL serological pipette, taking care not to break the aggregates into single cell suspensions. For example, for a 1:5 split ratio, resuspend the cell aggregates in 5 mL total PluriSTEM.
- Aliquot 1 mL of the appropriately diluted cell aggregates into the coated plates containing 2 mL PluriSTEM™ medium that had been set aside from step 2.
- Place the plate in a 37 °C incubator. Agitate the plate gently from side to side and forward and backwards to ensure that the cell aggregates are evenly distributed across the surface of the well. Incubate in a 37 °C incubator.
- After 10–15 minutes, visually inspect the plate to ensure that newly passaged cell aggregates are evenly distributed across the surface of the well.
- The next day, replace with 3 mL per well of fresh PluriSTEM™ medium.
- Monitor and exchange with 3 mL fresh PluriSTEM™ media daily. Depending upon the split ratio used, cells are typically ready for enzymatic passaging in 4–6 days.
- Cultures should be fed with 4 mL PluriSTEM™ media on Friday to allow sufficient media to sustain the cells over the weekend. Medium exchanges during the weekend are not necessary.
- When pluripotent cultures have been maintained in PluriSTEM™ media for at least 3 passages, media exchanges may be transitioned to every other day. Monitor cell health daily to ensure that every other day exchange does not affect the cell health and quality of the colonies.
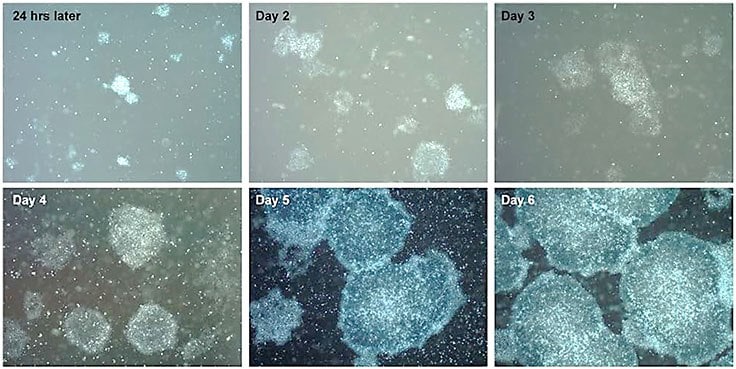
Figure 2. Time course of pluripotent stem cell expansion in PluriSTEM™ media.Human iPS cells expanded over a 6-day period in feeder-free conditions are ready to be passaged at day 6 when cells are 80% confluent.
Single Cell Passaging of Human ES/iPSCs in PluriSTEM™ Media
- On the day of passaging, acclimate ECM Gel Matrix (CC131) coated plates for 1 hour at room temperature. After 1 hour, remove the coating. Add 2 mL PluriSTEM™ media to each well. Set plate aside until cells are ready to be passaged.
- Aliquot sufficient PluriSTEM™ media (SCM130, SCM132), Accumax™ reagent (A7089) and DMEM/F12 (D6421) to passage the cells. Warm reagents at room temperature (15–25 °C) for 5–10 minutes.
- One hour before the cells are to be passaged, add ROCK Inhibitor, Y-27632 (SCM075) to each well of the 6-well plate to achieve a final concentration of 10 μM.
- After 1 hour, use a dissection microscope to visually inspect the plate containing human pluripotent cells to be passaged. Inspect the colonies for areas of spontaneous differentiation.
- Use a sterile p200 pipette tip attached to a p200 pipetman to scrape off areas of spontaneous differentiation. Be discriminating and scrape away any areas that harbor a hint of differentiation.
- Aspirate the medium containing the scraped areas from the well. Rinse with 2 mL per well with DMEM/F-12 medium or 1X PBS.
- Aspirate and replace with 1 mL of Accumax™ reagent per well of a 6-well-plate. Incubate at 37 °C for 8-10 minutes. Note: Different cell lines may require different incubation time. It is thus important to monitor the cell dissociation. Accumax™ treatment should be stopped when the cells start to dissociate and holes start to appear within colonies.
- Quench the Accumax™ reaction by adding 1 mL PluriSTEM™ media for each mL of Accumax™ reagent used. Gently detach cells using a sterile 1000-μL pipette tip. Cells should be easily dislodged.
- Collect the dissociated cells to a 15 mL conical tube. Rinse the wells with an additional 2 mL of PluriSTEM™ media to collect any remaining cells. Add the rinse to the 15 mL conical tube.
- Centrifuge the 15 mL conical tube containing the cell suspension at 300 x g for 5 minutes at room temperature (15–25 °C).
- Aspirate the supernatant. Resuspend the cells in fresh PluriSTEM™ media containing 10 μM ROCK Inhibitor, Y-27632.
- Count the number of cells using a Scepter™ Cell Counter or hemocytometer. Ensure that the cells are in a single cell suspension. Determine the cell viability using Trypan Blue exclusion.
- Set up a titration of different cell densities ranging from 0.5–1 x 104 cells/cm2. This corresponds to 50,000 – 100,000 cells per well of a 6-well plate in PluriSTEM™ media containing 10 μM ROCK Inhibitor, Y-27632.
- The next day, replace with fresh PluriSTEM™ media. The ROCK Inhibitor, Y-27632 is no longer required from this step forward. Replace with fresh PluriSTEM™ media daily (3 mL volume per well in a 6-well plate).
Results for Culture of Stem Cells in PluriSTEM™ Media
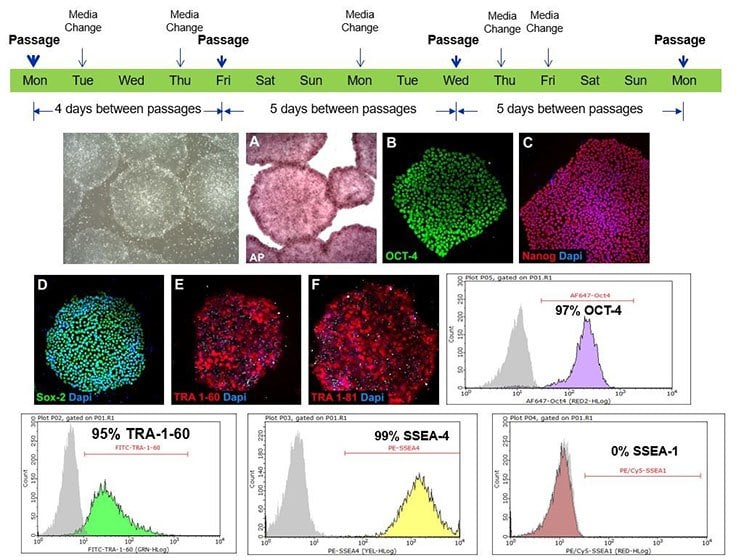
Figure 3. Pluripotent stem cells cultured in PluriSTEM™ media retain pluripotency markers.H9 human ES cells were maintained using an every-other-day/no weekend feeding schedule in PluriSTEM™ media for over 20 passages. Cells retained characteristic pluripotent morphology (i.e. homogeneous round colonies with defined borders) and high expression levels of alkaline phosphatase plus pluripotency markers Oct-4, Nanog, Sox-2, TRA-1-60, TRA-1-81, SSEA-4. Cells demonstrated an absence of staining for SSEA-1.
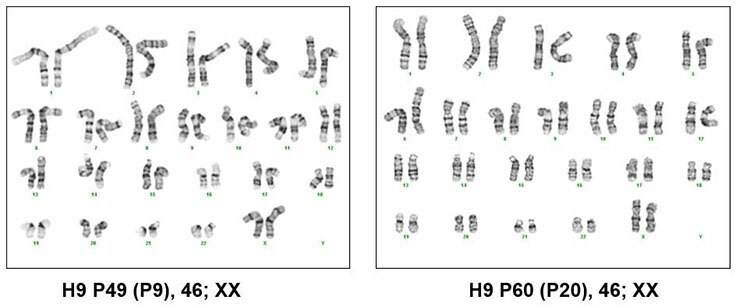
Figure 4. Karyotyping of pluripotent stem cells cultured in PluriSTEM™ media. H9 human ES cells maintained using an every-other-day/no weekend feeding schedule in PluriSTEM™ media for 9 and 20 passages have normal female karyotypes.
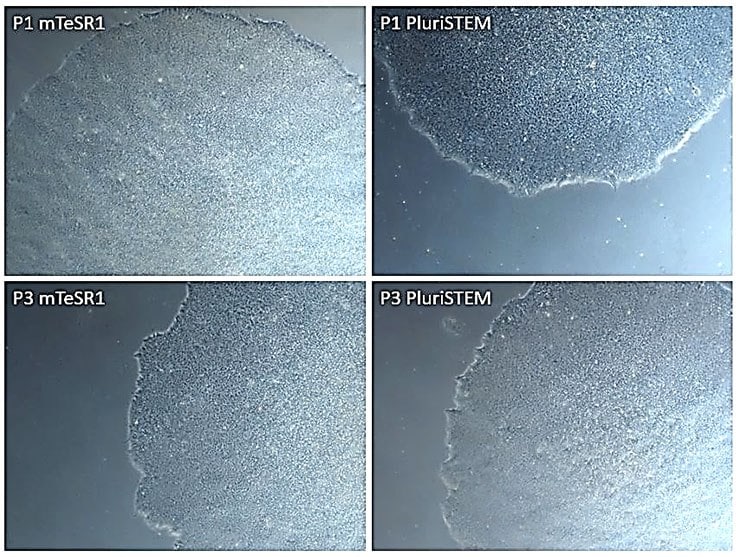
Figure 5. Human iPS cells cultured in PluriSTEM™ media vs. mTeSR1® media.Similar pluripotent morphologies were observed after 3 passages in both mTesr1® and PluriSTEM™ media.
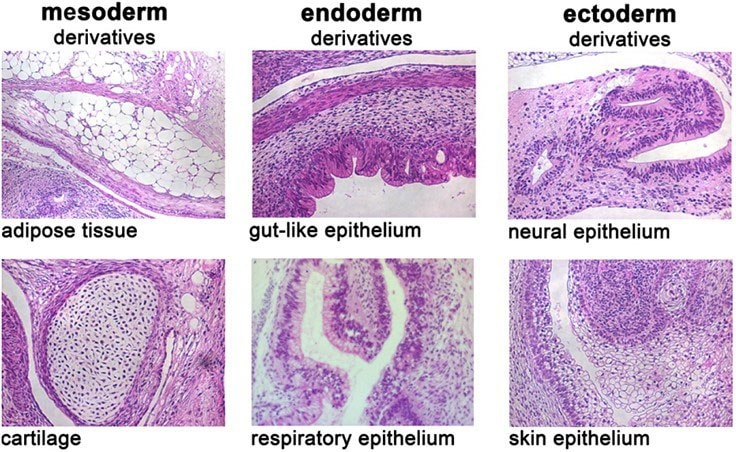
Figure 6. In vivo differentiation of pluripotent stem cells cultured in PluriSTEM™ media.Human pluripotent stem cells cultured in PluriSTEM™ media for >20 passages differentiate in vivo to expected germ layers. H&E stained teratoma sections courtesy of Dr. Boris Greber.
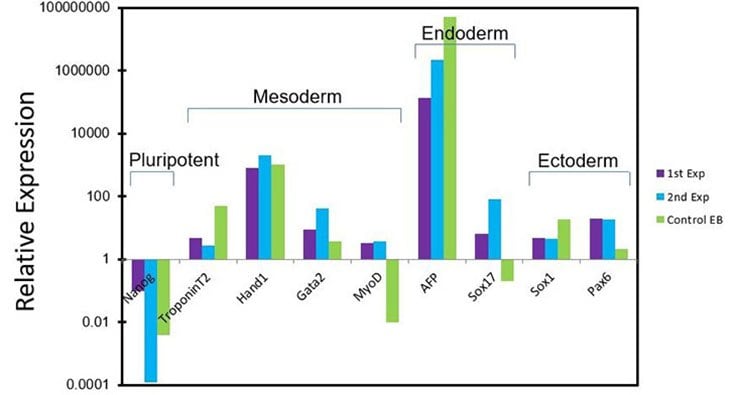
Figure 7. In vitro differentiation of pluripotent stem cells cultured in PluriSTEM™ media.Human pluripotent stem cells cultured in PluriSTEM™ media for >20 passages In vitro differentiate to all 3 germ layers (mesoderm, endoderm and ectoderm) using an embryoid body differentiation protocol.
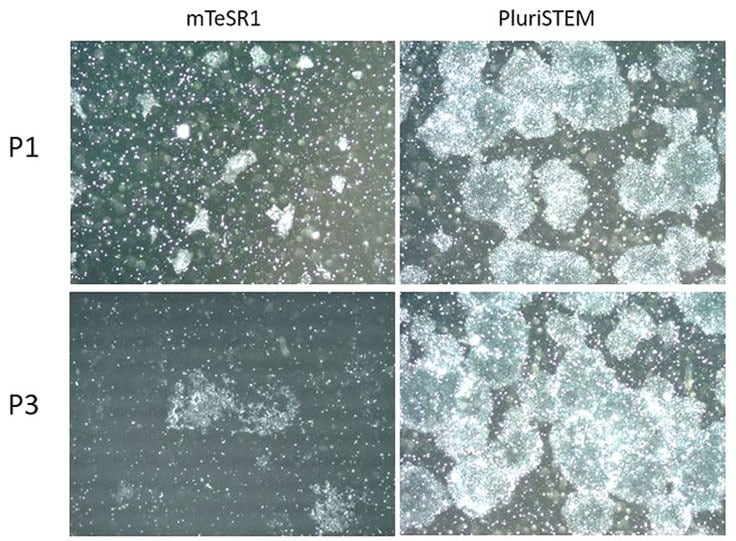
Figure 8. PluriSTEM™ media supports single cell passaging of pluripotent stem cells In vitro.H9 hESCs cultured in PluriSTEM™ media for 22 passages were dissociated into single cells using Accumax™ reagent. One hour before dissociation, 10 μM ROCK Inhibitor was added. 100,000 dissociated cells were seeded into each well of a coated 6-well-plate. Unlike cells cultured in mTesr1® media, single cells are highly viable in PluriSTEM™ media and continue to form pluripotent colonies after three rounds of single cell passaging using Accumax™ reagent.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?