Cationic Gold(I) Complexes Based on Bulky Phosphine Ligands: New Opportunity for Efficient Gold Catalysis
Introduction
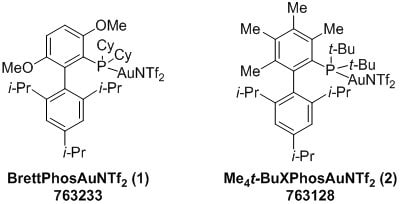
Highly sterically demanding phosphine ligands have proven to be essential in enabling many efficient transformations catalyzed by various late transition metals. The benefits of these ligands in homogenous gold catalysis have only recently been revealed. Below are the structures of two such sterically hindered gold catalysts, BrettPhosAuNTf2 (1) and Me4t-BuXPhosAuNTf2 (2), first reported by Prof. Liming Zhang.
Advantages
Several studies by Zhang show that cationic Au(I) complexes derived from highly hindered and electron-rich ligands such as BrettPhos and Me4t-BuXPhos are more effective catalysts than those typically derived from triarylphosphines and N-heterocyclic carbenes. Steric congestion around the gold center imposed by these ligands not only makes the electrophilic metal more stable, leading to longer catalyst lifetime, but also facilitates challenging reactions by limiting side reactions.
Representative Applications
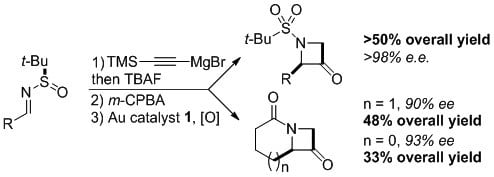
- In 2011, Zhang and coworkers demonstrated that enantioenriched azetidinones are readily accessible from Ellman's chiral sulfinimines in a 3-step protocol.1 BrettPhos-AuNTf2 (1) is particularly effective in catalyzing the final oxidative cyclization providing these small nitrogen heterocycles in up to 99% ee.
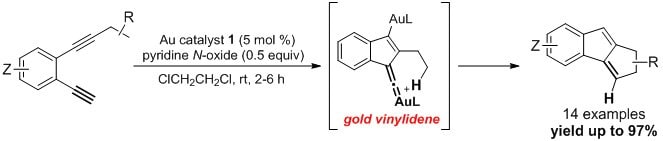
- Cationic gold complex 1 is optimal for the construction of 1,2-dihydrocyclopenta[a]indenes from diynes, where a novel gold vinylidene species is invoked based on experimental and theoretical studies.2 Other catalysts such as IPrAuNTf2 are substantially less effective.
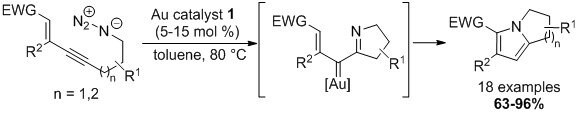
- BrettPhosAuNTf2 (1) is the best in catalyzing an intramolecular nitrene transfer by using an azido group as the nitrene source.3 The bicyclic pyrroles formed are valuable synthons, as demonstrated by a formal synthesis of 7-methoxymitosene.
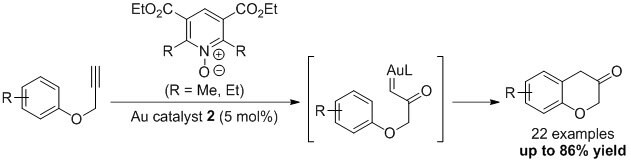
- With the most hindered gold complex Me4t-BuXPhosAuNTf2 (2), a strategy based on intermolecular gold-catalyzed oxidation of an alkyne offers rapid access to chroman-3-ones from readily available propargyl aryl ethers.4
*Special thanks to Prof. Liming Zhang for contributing this technical spotlight.
References
To continue reading please sign in or create an account.
Don't Have An Account?