Complex Hydrides: New Solid-state Conductors
Motoaki Matsuo1, Hiroyuki Oguchi1,2, Hideki Maekawa2, Hitoshi Takamura2, Shin-ichi Orimo1
1Institute for Materials Research, Tohoku University Katahira 2-1-1, Sendai, 980-8577, Japan, 2Graduate School of Engineering, Tohoku University Aramaki Aza Aoba 6-6-02, Sendai, 980-8579, Japan
Introduction
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes. So far, a wide variety of inorganic lithium fast-ion conductors of oxides,1 ,2 nitrides3 and sulfides4, 5 have been extensively studied because they have an advantage in high ion conductivity [>10-3 S/cm at room temperature (RT)] as well as high lithium ion transport number (~1). Further efforts continue to overcome various barriers to practical realization, for example very high grain boundary resistance.
Lithium fast-ion conduction was discovered in LiBH4 (686026) in 2007. LiBH4 has not only high ion conductivity but also the following advantages for the solid electrolyte in lithium-ion batteries: (1) extremely low grain boundary resistance, (2) high electrochemical stability up to at least 5 V (vs Li+/Li) at 390 K, (3) extremely low polarization to metallic electrodes, (4) commercial availability and (5) suitability for various material processing techniques, including mechanical milling, heat treatment, impregnation and vapor deposition. This article reviews the fast-ion conduction in LiBH4 and its derivatives (LiBH4-LiI and LiBH4-LiNH2 systems), as a new category of solid-state lithium fast-ion conductors.
Lithium Fast-ion Conduction in LiBH4
Complex hydrides are generally expressed as M(M′Hn), where M is a metal cation and (M′Hn) represents a complex anion such as (NH2)-, (BH4)- and (AlH4)-. LiBH4 is a representative complex hydride, and has recently attracted great interest as a potential candidate for advanced hydrogen storage materials because of its high hydrogen density. LiBH4 undergoes a structural transition at about 390 K from the orthorhombic low-temperature (LT) phase to the hexagonal high-temperature (HT) phase. Our study of microwave irradiation-induced rapid hydrogen desorption suggested that LiBH4 might become conductive in the HT phase, although it is an insulator in both the LT phase and the HT phase with large band gap of about 7 eV.
Therefore, we systematically examined the lithium ion conductivity of LiBH4 by an arc complex impedance method and 7Li NMR measurement.6
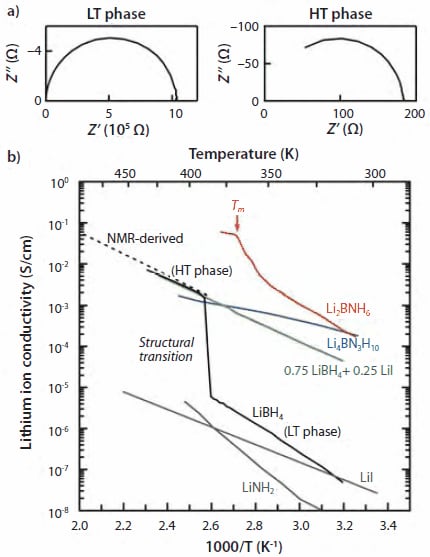
Figure 1. Electrical properties of LiBH4 and its derivatives. (a) Typical impedance plots for the LT phase and the HT phase of LiBH4 obtained using lithium-metal electrodes. (b) Temperature dependence of the lithium ion conductivity. The melting temperature of Li2BNH6, 368 K, is indicated as Tm.
Figure 1(a) shows the temperature dependence of the electrical conductivity of LiBH4 as determined from the impedance plots shown in Figure 1(b). The impedance plots of both the LT phase and the HT phase show only a single arc, indicating that the response arising from the grain boundary is not observed, although the pelletized sample used for the impedance measurement was prepared simply by pressing powder LiBH4 without subsequent sintering. The conductivities of the LT phase are very low, in the range of 10-8 to 10-6 S/cm, and the value linearly increases with increasing temperature. At about 390 K, the transition temperature, the conductivity drastically increases by three orders of magnitude. As a result, the HT phase exhibits high conductivity of the order of 10-3 S/cm. The activation energies for conduction are evaluated to be 0.69 eV and 0.53 eV for the LT and HT phases, respectively.
In order to confirm whether the high electrical conductivity of LiBH4 in the HT phase is due to the lithium fast ion mobility, 7Li NMR was measured. Figure 2 shows the 7Li NMR spectra at selected temperatures.
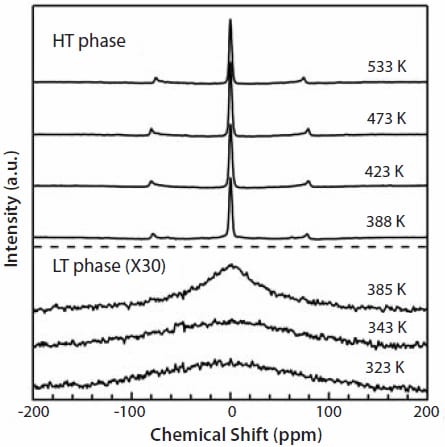
Figure 2. 7Li NMR spectra of LiBH4 at selected temperatures.
The shapes of the spectra drastically change at the structural transition temperature. In the LT phase (<385 K), only broad and small peaks are observed. On the contrary, in the HT phase (>388 K), each spectrum shows a central sharp line and two satellite lines. The decrease in the line width of the central line indicates the motional narrowing caused by the lithium fast ion motion in the HT phase. Furthermore, Figure 1 also shows the NMR-derived conductivity estimated by the Nernst- Einstein equation using the correlation times obtained by the temperature dependence of the spin-lattice relaxation time T1. Clearly, the NMR data show a fairly good agreement with that measured by the impedance method. Thus, we concluded the charge carrier must be Li+ ions; the HT phase is a lithium fast-ion conductor.
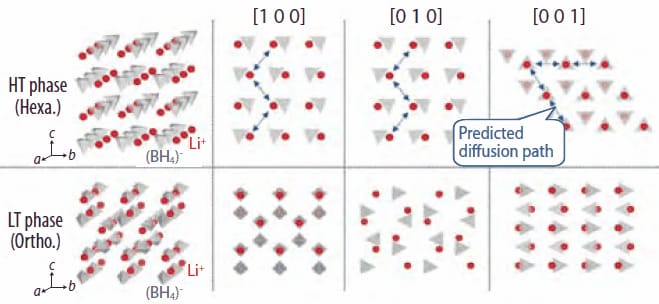
Figure 3. Crystal structures of LiBH4 in orthorhombic LT phase (bottom) and hexagonal HT phase (top). Red sphere and gray tetrahedrons show Li+ and (BH4)- ions, respectively. Blue arrowed lines show the predicted diffusion path.
As shown in Figure 3, the characteristic feature of the HT structure is that both Li+ ions and (BH4)- ions line up along the a axis and the b axis such that there is no (BH4)- ion between Li+ ions and vice versa. This arrangement may enable Li+ ions to migrate along these directions. Detailed studies using first-principles molecular dynamics simulations7 and high-pressure impedance measurements8 are now under way.
Enhanced Conductivity of the Complex Hydrides Derived from LiBH4
The lithium fast-ion conduction in LiBH4 could potentially aid the development of solid electrolytes in all-solid-state batteries. From an application point of view, however, it is highly desirable to enhance the conductivity at room temperature (RT). For that purpose, next, we tried materials design of fast-ion conductors of complex hydrides. As a typical example, we have investigated the LiBH4-LiI and LiBH4-LiNH2 systems with different concepts.
Stabilization of the HT Phase in the LiBH4-LiI System9-11
We assumed that the stabilization of the HT phase should significantly improve the conductivity at RT, and the substitution of (BH4)- ion by I- ion, of which ion radius (2.20 Å) is larger than that of (BH4)- ion (2.05 Å), might be effective for that purpose because it has been reported that alkali-metal borohydrides MBH4 (M = Na, K, Rb and Cs) with longer distances between neighboring (BH4)- ions exhibit the lower transition temperatures.12
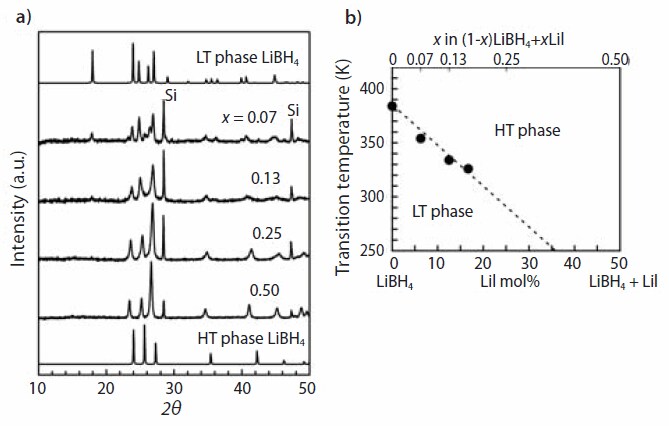
Figure 4. (a) XRD profiles of (1–x)LiBH4 + xLiI synthesized by mechanical milling (x = 0.07 - 0.50) and (b) structural transition temperatures determined by DSC as a function of value x (top axis) and “LiI mol %” (bottom axis).
Figure 4(a) shows the x-ray diffraction (XRD) profiles of (1-x)LiBH4 + xLiI synthesized by mechanical milling. The profiles gradually change from the LT phase to the HT phase with increasing amounts of LiI (518018) . For x = 0.25 and 0.5, only the peaks corresponding to the HT phase are observed with being shifted to lower angle, indicating the substitution of (BH4)- ion by I- ion. The transition temperatures determined by differential scanning calorimetry (DSC), shown in Figure 4(b), clearly show the stabilization feature of the HT phase depending on the molar ratio of LiI. The vibrational properties measured by spatially resolved Raman spectroscopy demonstrated the importance of anharmonic effects in the structural transformation in this system.13
Figure 1 also shows the result of the ion conductivity measurements for x = 0.25. The conductivity follows the Arrhenius behavior throughout the measured temperature range without the abrupt change observed for LiBH4 due to the structural transition. As expected, we achieved the increase in the conductivity at RT by 3 orders of magnitude without high conductivity of the HT phase decreased. We also confirmed that the substitution of (BH4)- ion by Cl- ion leads to the enhanced conductivity.14
Double Complex Anions in the LiBH4-LiNH2 System15
The LiBH4-LiNH2 system has been studied from the viewpoint of developing hydrogen storage materials, and two phases, Li2BNH6 and Li4BN3H10, have been known to exist. As shown in Figure 5, both Li2BNH6 and Li4BN3H10 have different configuration of anions from LiBH4: Li+ ions are tetrahedrally coordinated by combinations of (BH4)- and (NH2)-. The LiBH4-LiI system showed the ion conductivity is affected by configuration of anions to a great degree, which made us get interested in these local structures.
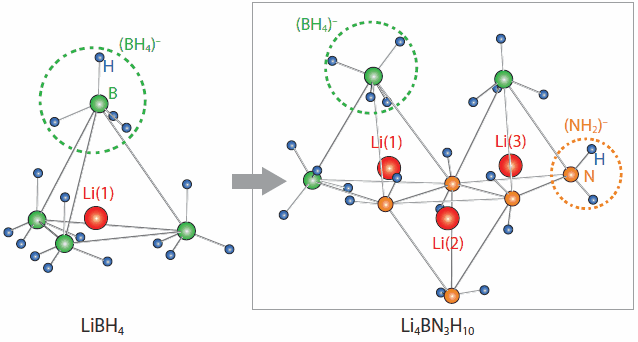
Figure 5. Local atomic structures of LiBH4 (left) and Li4BN3H10 (right). Red, green, orange and blue solid spheres show Li, B, N and H, respectively. Li4BN3H10 has multiple occupation sites for Li+ ions while LiBH4 has only one site.
The conductivity measurements indicate that Li2BNH6 has fast-ion conductivity of 2×10-4 S/cm at RT, which is 4 and 5 orders of magnitude higher than those of the host hydrides LiBH4 and LiNH2, respectively, and the conductivity monotonically increases upon heating (Figure 1). The activation energy significantly decreases at around 368 K from 0.56 eV (303-348 K) to 0.24 eV (above 368 K) due to melting. The total ion conductivity reaches up to 6×10-2 S/cm at the highest temperature measured 378 K. Li2BNH6 could be a good ionic liquid electrolyte as well as a solid electrolyte. Li4BN3H10 also exhibits high ion conductivity of 2×10-4 S/cm even at RT. The activation energy is evaluated to be 0.26 eV. It is noteworthy that this value is less than half those in Li2BNH6 before melting and LiBH4, indicating that Li4BN3H10 has higher lithium ion mobility. This feature was also confirmed by 7Li NMR, reflecting the different configuration of anions.15
Future Perspective
In this short review, we have introduced the lithium fast-ion conduction in LiBH4 and its derivatives. We have recently elucidated that (NH2)-based and (AlH4)-based complex hydrides are also lithium ion conductors. The further research and development should provide new lithium ion conductors and new phenomena characteristic for complex hydrides.
Additionally, our group is currently focusing on the following subjects in order to demonstrate all-solid-state batteries using complex hydride solid electrolytes:
- Search for non-oxide cathode materials
- Synthesis of thin-film Li4BN3H10
Among the complex hydrides introduced in this review, Li4BN3H10 is the most promising candidate for solid electrolyte because it has the highest conductivity and the lowest activation energy. Thin-film Li4BN3H10 would allow the fabrication of thin-film lithium ion batteries, such as graphite/thin-film Li4BN3H10/above-mentioned new cathode, for micro electromechanical system (MEMS).
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?