Determination of Omeprazole and Related Compounds Using a High pH Stable Superficially Porous Particle C18 Column
Abstract
Section Overview
Introduction
Omeprazole is used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger–Ellison syndrome. It has been included in the World Health Organization list of Essential Medicines and is commercially available as a generic formulation. In 2022, it was reported as the ninth most prescribed medication in the United States, with more than 52 million prescriptions.
The selectivity of an HPLC separation largely depends on the interaction between the analyte and the stationary phase. To ensure optimal interactions for a separation, it is often required to adjust the pH of a mobile phase to a more basic level and above the common range for silica-based columns (2–8). These high pH values can then damage the particle structure and lead to bad peak shapes and insufficient resolution, resulting in unstable or irreproducible methods.
Because omeprazole is a basic compound that is sensitive to pH-driven changes in ionization, high pH stability of the stationary phase is crucial for maintaining omeprazole's ionization state, ensuring its stability, enhancing separation efficiency, and achieving reproducible results. This study emphasizes the benefits of using a high pH stable stationary phase for the accurate and reliable determination of omeprazole and its related compounds.
The Ascentis® Express 120Å C18 pH+ column, which operates within a pH range of 2 to 12, enables effective method development and improved separation of basic compounds that exhibit poor peak shapes and insufficient retention at lower pH. The column is designed for high pH mobile phases and prevents silica particle degradation, as demonstrated by thorough stability testing at elevated temperatures. With a 120 Å pore size and the use of superficially porous particle (SPP) Fused Core® technology, this column demonstrates optimum performance under high pH conditions and provides improved separation speed and chromatographic efficiency.
Experimental
The HPLC separation of omeprazole requires the use of a high pH stable environment because its chemical properties are strongly influenced by pH. It is classified as a proton pump inhibitor and exhibits marked sensitivity to pH, which affects its ionization, solubility, and stability during analysis.
With a pKa of approximately 4.0, omeprazole remains predominantly protonated at low pH and becomes progressively deprotonated as the pH increases. This change in ionization alters its interaction with the stationary phase and consequently affects the retention behavior and separation efficiency. Omeprazole is also unstable under acidic conditions, which makes the maintenance of a controlled and stable higher pH necessary to prevent analyte degradation and to ensure accurate quantification.
Chromatographic Conditions
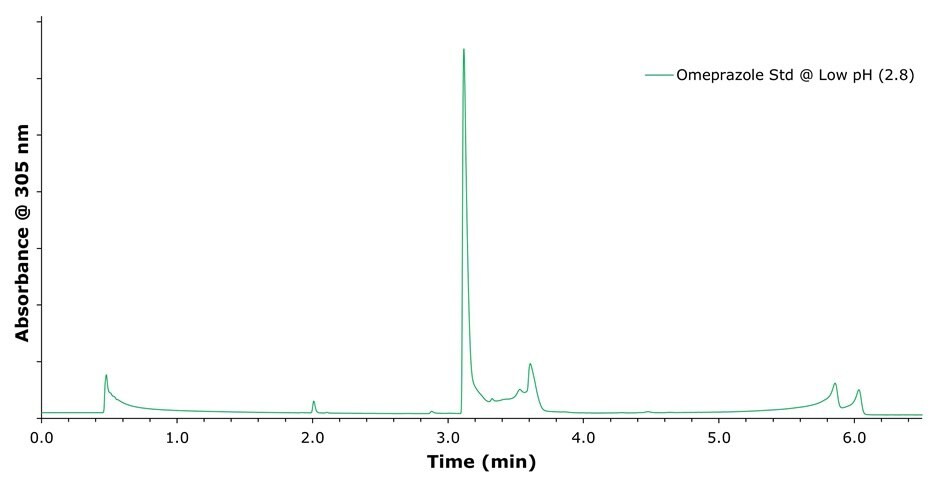
Figure 1.Chromatographic separation of omeprazole standard solution using a mobile phase consisting of 0.1% formic acid at pH 2.8 and acetonitrile under gradient elution. (see chromatographic conditions Table 1)
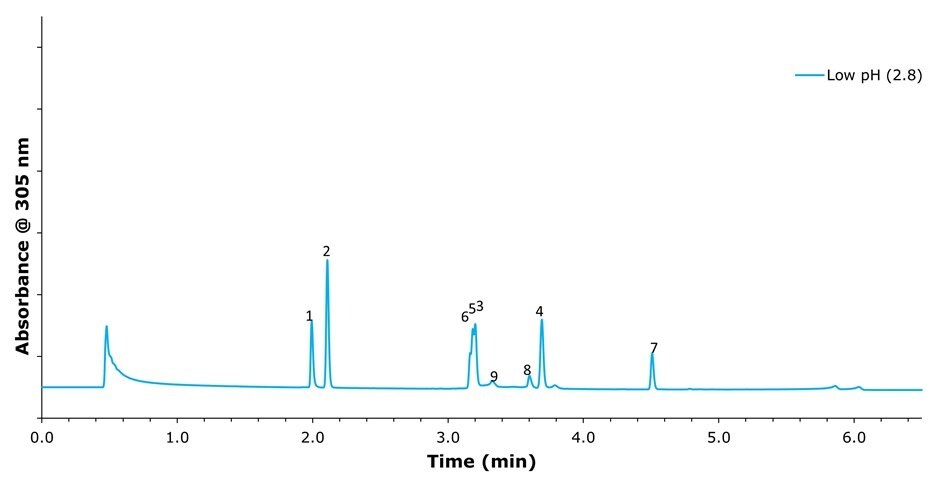
Figure 2.Chromatographic separation of omeprazole and its related compounds using a mobile phase consisting of 0.1% formic acid at pH 2.8 and acetonitrile under gradient elution. Peak IDs: (1) related compounds F and G; (2) related compound B; (3) related compound E; (4), related compound A; (5) impurity B; (6) Omeprazole; (7) impurity H; (8) N'-methyl Omeprazole; (9) impurity C.
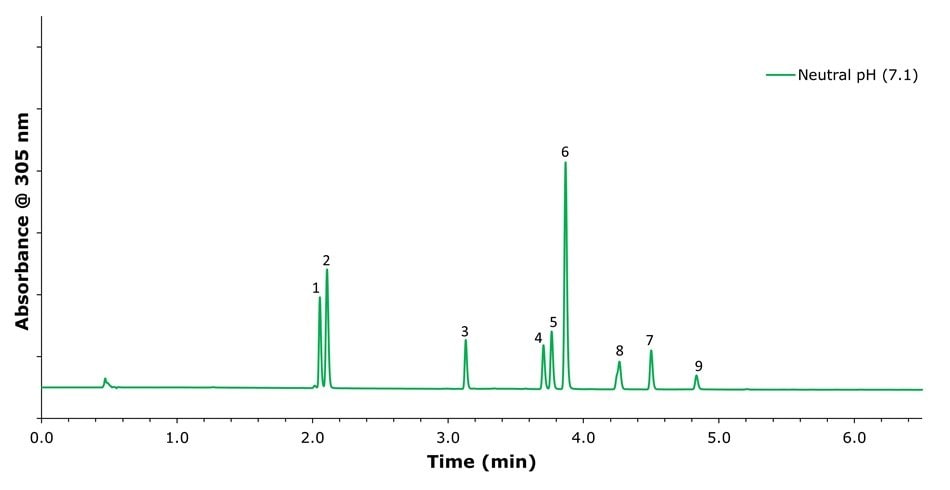
Figure 3.Chromatographic separation of omeprazole and its related compounds using a mobile phase consisting of 20 mM potassium phosphate buffer at pH 7.1 and acetonitrile under gradient elution. Peak IDs: (1) related compounds F and G; (2) related compound B; (3) related compound E; (4), related compound A; (5) impurity B; (6) Omeprazole; (7) impurity H; (8) N'-methyl Omeprazole; (9) impurity C.
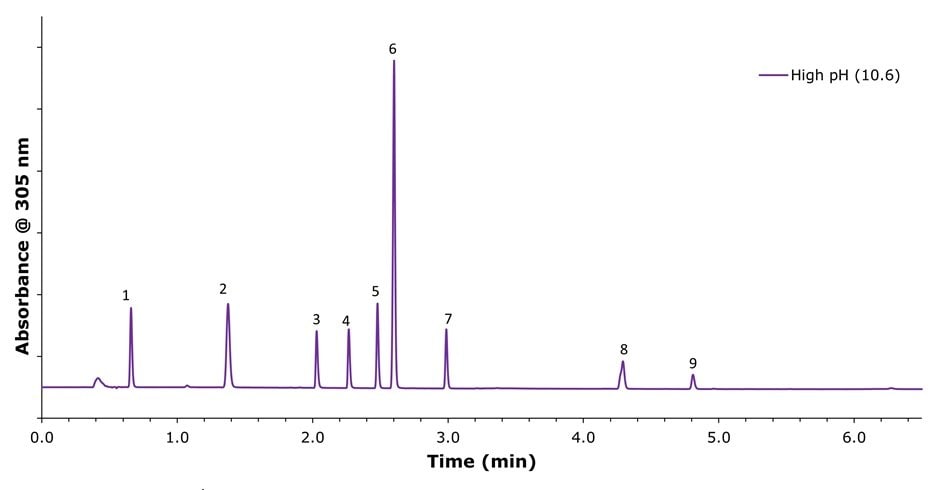
Figure 4.Chromatographic separation of omeprazole and its related compounds using a mobile phase consisting of 0.03% ammonium hydroxide in water at pH 10.65 and acetonitrile under gradient elution. Peak IDs: (1) related compounds F and G; (2) related compound B; (3) related compound E; (4), related compound A; (5) impurity B; (6) Omeprazole; (7) impurity H; (8) N'-methyl Omeprazole; (9) impurity C.
Mobile phase considerations
Acetonitrile is widely used in high performance liquid chromatography, particularly in pharmaceutical and environmental analyses, because it provides favorable chromatographic performance. Methanol, however, offers notable sustainability advantages due to its lower toxicity and suitability for greener applications.
Methanol is considered more environment friendly because it is biodegradable and can be produced from renewable resources, which contributes to a reduced carbon footprint. Additionally, methanol is typically less expensive and more readily available, especially with the increasing production from renewable sources. This makes methanol an attractive alternative for HPLC applications. .
In this study, the organic component of the mobile phase was replaced with methanol to improve the sustainability profile of the method (Table 2, Figure 5).
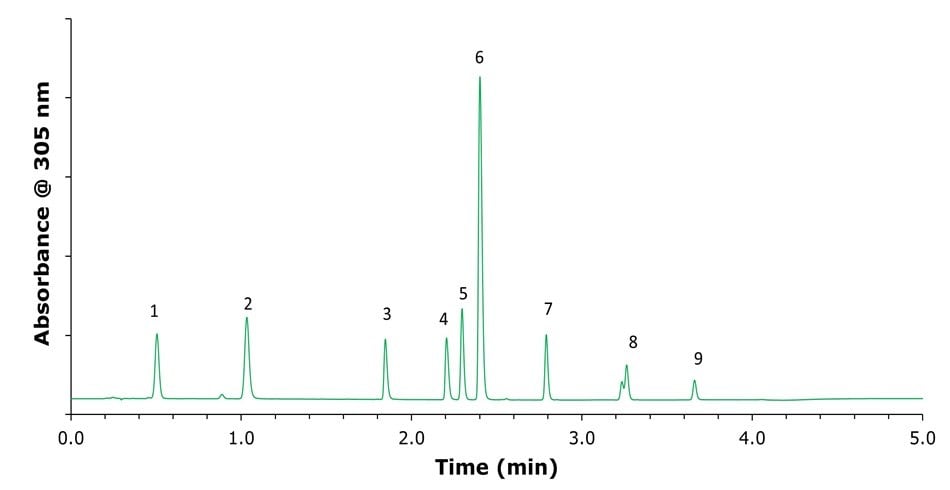
Figure 5.Chromatographic separation of omeprazole and its related compounds using a mobile phase consisting of 0.03% ammonium hydroxide in water at pH 10.65 and methanol under gradient elution. Peak identities, (1) related compounds F and G; (2) related compound B; (3) related compound E; (4), related compound A; (5) impurity B; (6) Omeprazole; (7) impurity H; (8) N'-methyl Omeprazole; (9) impurity C.
Column comparison
A direct comparison of several commercially available high performance liquid chromatography columns revealed clear differences in selectivity (Figure 6). The comparison was performed using a mobile phase consisting of 0.03% ammonium hydroxide buffer at pH 10.65 and methanol under gradient elution.
The Ascentis® Express 120 Å C18 pH+ column provided the highest resolution for all evaluated compounds and produced well-defined peak shapes. Under these chromatographic conditions, in which methanol was used as the organic component, the N'-methyl omeprazole peak (8) showed splitting into its isomeric forms on all tested columns.
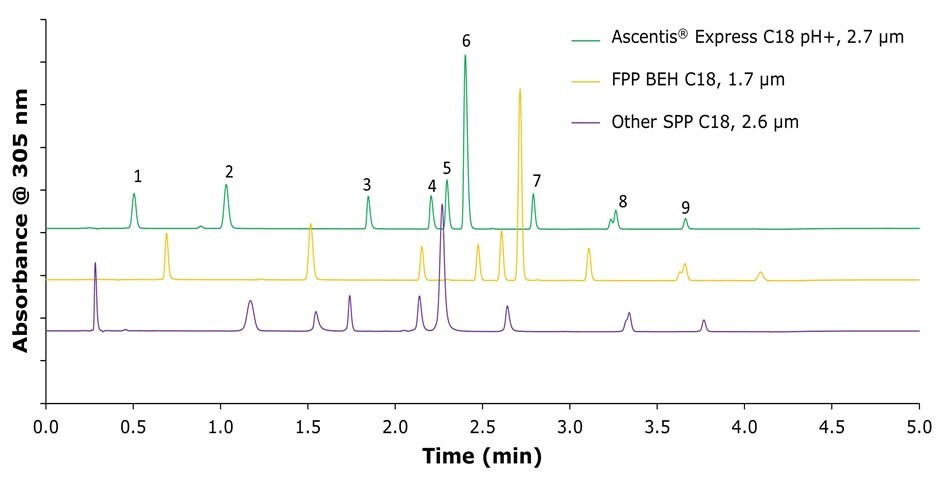
Figure 6.Comparison of three commercially available HPLC columns (50 × 2.1 mm) for the separation of omeprazole and its related compounds using a mobile phase consisting of 0.03% ammonium hydroxide in water at pH 10.65 and methanol under gradient elution.
Stability
The Ascentis® Express 120 Å C18 pH+ columns demonstrated excellent long-term stability under high pH conditions (Figure 7). This durability supports the development of robust chromatographic methods and prolongs column lifetime, even under conditions that typically lead to degradation of conventional silica-based HPLC columns.
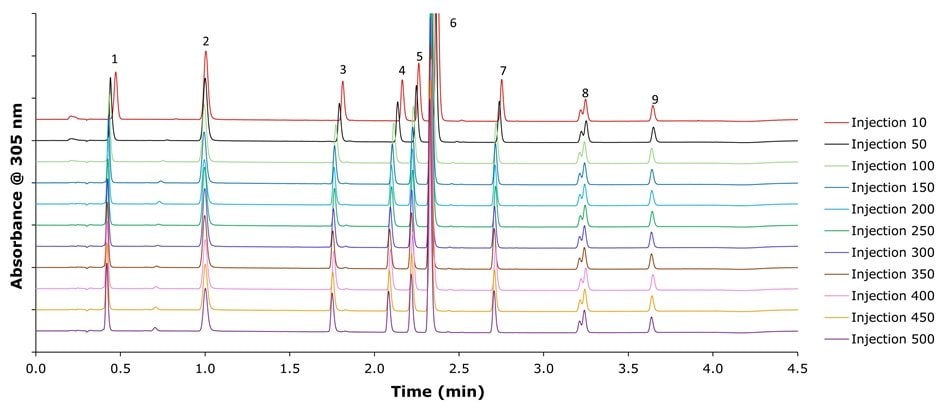
Figure 7.Stability of Ascentis® Express 120 Å C18 pH+ (2.7 μm), 50 mm x 2.1 mm I.D. column for the separation of omeprazole and related compounds with a mobile phase of 0.03% ammonium hydroxide at pH 10.65 and methanol by gradient elution over 500 sample injections with 34,482 column volumes in total.
Certified Reference Materials
To continue reading please sign in or create an account.
Don't Have An Account?