Glycol Analysis in Cough Syrup by GC-MS Using an Equity-1701 Capillary GC Column
Abstract
Gas chromatography coupled to mass spectrometry detection (GC-MS/MS) was used as an analytical tool to analyze glycols present in commercial cough syrup samples with an Equity-1701 Capillary GC Column 30 m × 0.25 mm, df 0.25 μm. The developed method is able to separate and quantify ethylene glycol, propylene glycol, diethylene glycol, glycerin, triethylene glycol, and sorbitol present in the commercial cough syrup preparations. For the quantification, certified reference materials (CRMs) were used. The method is partially validated for establishing the performance characteristics.
Section Overview
Introduction
Glycols are chemical compounds characterized by two hydroxyl groups attached to separate carbon atoms, commonly referred to as aliphatic diols.1 Various forms of glycols are utilized in industries for different purposes, such as liquid desiccants in air conditioning systems, antifreeze in car radiators2, and are also used as additives in hydraulic and brake fluids. Ethylene glycol is recognized as the simplest type. Diethylene, triethylene, and propylene glycols are oligomers of ethylene glycol. Ethylene glycol is a potent cause of acute toxicity in humans, in contrast, propylene glycol is a generally recognized as safe additive for foods and medications.3 Propylene glycol is generally considered safe, but when used in high doses or used for prolonged periods, propylene glycol toxicity can occur with reported adverse effects includes central nervous system (CNS) toxicity, hyperosmolarity, hemolysis, cardiac arrhythmia, seizures, agitation, and lactic acidosis.4 Typically, colorless, and odorless, ethylene glycol and diethylene glycol have a sweet taste5 and are often considered inexpensive adulterants, making them attractive for commercial sectors involved in drug formulation, including pediatric drugs.
However, when glycols are added to drug formulations beyond recommended limits, they can be toxic to human health, potentially leading to acute hepatotoxicity, renal failure, and even death in some cases. In recent times fatal incidences were reported e.g. from Indonesia and Gambia where of ethylene glycol and diethylene glycol were found in syrup medicines. 6,7,8 As a response the US Food and Drug Administration (US FDA) provided a guidance for high-risk drug components9 and the United States Pharmacopoeia (USP) revised the monograph for propylene glycol that addresses the toxicity concerns by specifying the limits for ethylene glycol and diethylene glycol in formulation components such as propylene glycol to be not more than (NMT) 0.1% to ensure quality standards for pharmaceutical products.10
This article will present a method for the simultaneous determination of five glycols and sorbitol in a cough syrup sample using GC-MS/MS. Two commercially available cough syrups are analyzed unspiked and spiked. The developed method was partially validated as per the ICH recommended guidelines11 and used for determination of minimum limit of detection and quantification of glycols. Furthermore, two commercially available cough syrups are analyzed and compared to the occupational health and safety limits and permissible limits in drug products stated by USP.
The Equity-1701 column fulfils USP G46 requirements with 14% cyanopropylphenyl - 86% dimethylpolysiloxane and the max temperature limit of this column is 280 ̊C. Even though this column is from the mid polar range, the Equity‑1701 stationary phase along with its temperature characteristics was found capable for additional determination of sorbitol along with other glycol present in the samples. Since Sorbitol is exhibiting a very high boiling point of 296 °C, in many cases, the reported method of choice for sorbitol is HPLC-RI/ELSD. Here it was included as additional analyte for the GC-MS/MS technique to further explore the capability of the developed method on the selected Equity-1701 phase.
Structures of analytes in this study.
Experimental
Standard preparation
The glycol standards were prepared as single component solutions of ethylene glycol (EG), propylene glycol (PG), diethylene glycol (DEG), glycerin (Gly), triethylene glycol (TEG), Sorbitol (SOR) according to the following procedures using certified reference materials (CRMs) and methanol/water (95:5, v/v) as diluent.
- Stock solutions glycols (500 µg/mL): Weigh and transfer about 10 mg of glycol substance into a 20 mL volumetric flask, add about 10 mL of diluent and sonicate for 5 minutes, make up to volume with diluent and shake to mix thoroughly. The concentration of the glycol in the resulting solution is 500 µg/mL.
- Stock solutions sorbitol (1000 µg/mL): Weigh and transfer about 10 mg of sorbitol into a 10 mL volumetric flask, add about 10 mL of diluent and sonicate for 5 minutes, make up to volume with diluent and shake to mix thoroughly. The concentration of the sorbitol in the resulting solution is 1000 µg/mL.
- Standard solution (SS, all glycols 20 µg/mL, sorbitol 200 µg/mL): Transfer 400 µL of each glycol stock solution and 2 mL of Sorbitol stock solution into a 10 mL volumetric flask, add 5 mL of diluent and sonicate for 5 minutes. Then make up to volume with diluent and mix thoroughly.
Calibration Solutions
Calibration solutions were prepared as single-component solutions using the following dilution schemes:
Transfer of 100, 250, 500, 750, and 1000 µL from each of the above-mentioned glycol standard stock solutions of ethylene glycol, propylene glycol, diethylene glycol, glycerin and triethylene glycol into 10 mL volumetric flask individually and to each individual flasks add 2, 2.5, 3, 3.5 and 4 mL of sorbitol stock solution and make up to volume with diluent, sonicate for 5 minutes and mix thoroughly. Resulting solutions have 5.0, 12.5, 25.0, 37.5, and 50.0 µg/mL for each glycol and 200.0, 250, 300, 350, and 400 µg/L sorbitol respectively.
Sample Preparation
Transfer 12 mg of commercially available cough syrup into a 10 mL volumetric flask, add 5 mL of diluent and sonicate for 5 minutes. Fill up to mark with diluent and mix thoroughly. Two commercially available syrups (A & B) were used for the assessments.
Spiked Samples
- Spiked sample 1 PG & Gly (20.8 mg/g): Weigh 12 mg of syrup sample A into a 10 mL volumetric flask and add 500 µL of each of PG and Gly stock solutions. Make up to mark with diluent, mix thoroughly and sonicate for 5 minutes. Solution contains 25 µg/mL of each glycol.
- Spiked sample 2 PG & Gly (41.7 mg/g): Weigh 12 mg of syrup sample A to a 10 mL volumetric flask and add 1000 µL of each of PG and Gly stock solutions. Make up to mark with diluent, mix thoroughly and sonicate for 5 minutes. Solution contains 50 µg/mL of each glycol.
- Spiked Sample 3 PG, Gly (20.8 mg/g): Weigh 12 mg of syrup sample B into a 10 mL volumetric flask and add 500 µL of each of PG and Gly stock solutions. Make up to mark with diluent, mix thoroughly and sonicate for 5 minutes. Solution contains 50 µg/mL of each glycol.
- Spiked sample 4 PG, Gly (41.7 mg/g): Weigh and transfer 12 mg of commercially available sample B to a 10 mL volumetric flask, and add 1000 µL of each of PG, Gly stock solutions. Make up to mark with diluent, mix thoroughly and sonicate for 5 minutes. Solution contains 50 µg/mL of each glycol.
GC-MS Analysis
The GC-MS analysis was performed on an intermediate polarity Equity-1701 column (Table 1). Depending upon the fragmentation recorded for each individual glycols, the selection of specific target ions and reference ions were selected for scanning at a defined retention time. Selection of the retention time was done as per the separation acquired by the developed method on Equity-1701 column (Table 2).
Results and Discussion
The developed method applying an Equity-1701 (30 m × 0.25 mm I.D., df 0.25 μm) capillary GC column and splitless injection showed good retention and sufficient resolution of the analytes in scope. A representative chromatogram of an analyte mixture at 20 µg/mL each (sorbitol at 200 µg/mL) is shown in Figure 1.
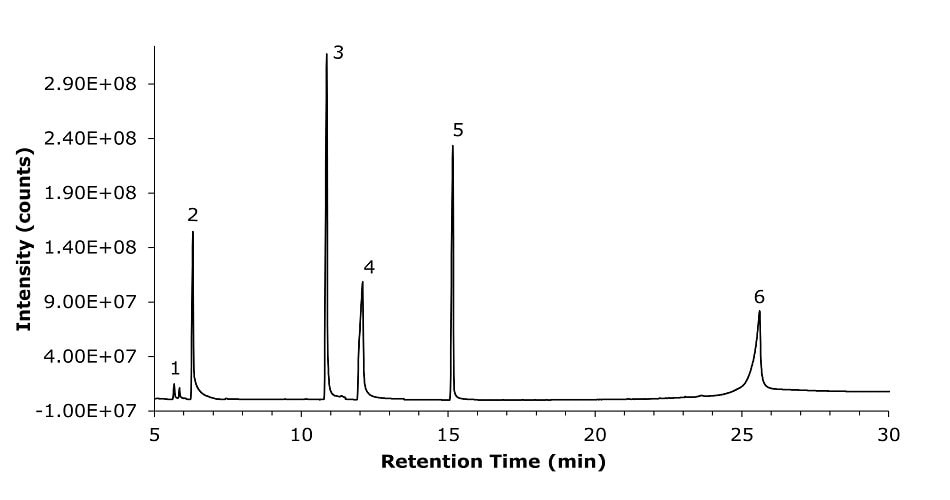
Figure 1.GC-MS chromatogram (SIM) for the standard solution (SS) mixture containing ethylene glycol (1), propylene glycol (2), diethylene glycol (3), glycerin (4), triethylene glycol (5), prepared in diluent at 20 µg/mL and sorbitol (6) at 200 µg/mL.
Calibration
The quantification was based on an external calibration using single reference standard solutions as described in the experimental section. The GC-MS method linearity for ethylene glycol, propylene glycol, diethylene glycol, glycerin and triethylene was established in the concentration range of 5 to 50 μg/mL, and for sorbitol 200 to 400 µg/mL. In Tables 3 & 4, the experimental results including the linearity (R2) and the limits of detection (LOD) and limits of quantification (LOQ) are summarized for all analytes. Based on the developed method the minimum concentrations of glycols that can be detected in the injected solutions as per the ICH guidelines11 by using this method’s linearity study is ranging for the five glycols from 3.33 µg/mL to 4.49 µg/mL, for sorbitol 50.12 µg/mL, and the quantification can be done in the range of 10.09 µg/mL to 13.6 µg/mL, for sorbitol 126.58 µg/mL. Derived from the linearity study data the estimated potential LODs of glycols present in the original cough syrup samples are in the range of 2.78 mg/g to 3.74 mg/g, for sorbitol 41.74 mg/g, and the LOQs in the range of 8.41 mg/g to 11.33 mg/g, for sorbitol 126.58 mg/g.
Reproducibility
The system repeatability was demonstrated by injecting five replicates of the standard solution (SS) containing mixture of glycol standards of ethylene glycol (EG), propylene glycol (PG), diethylene glycol (DG), glycerin (Gly), triethylene glycol (TG) and sorbitol (SOR) prepared in diluent (see Figure 1 and for diluent blank Figure 2) The determined RSD ranged from 3.63-8.89% (Table 5).
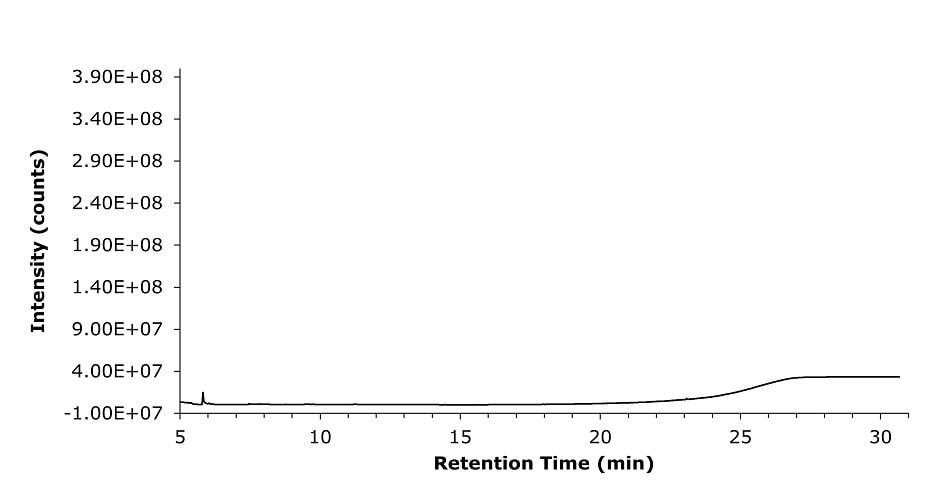
Figure 2.GC-MS chromatogram recorded for diluent (blank) containing methanol and water (95:5 v/v).
Recovery Determination
For recovery determinations four solutions were prepared with 2 spike concentration levels 20.8 µg/g and 41.7 µg/g (25 and 50 µg/mL in the injection solutions) and two sets of compounds (Table 6 & 7) and 2 syrups used (A&B). For recovery calculation existing glycol background was subtracted.
Original Sample Analysis
The method was applied for analyzing two different commercially available cough syrup samples (A & B) solutions (see Figures 3 & 4). Table 8 shows the results for the analysis of glycols present in the two commercially available cough syrup samples (A & B).
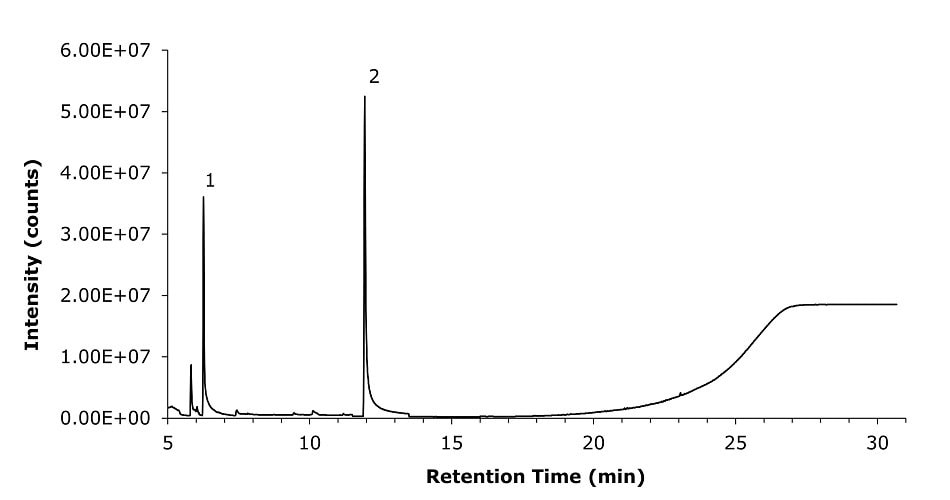
Figure 3.GC-MS chromatogram recorded for commercially available cough syrup sample A prepared in diluent, showing the presence of propylene glycol (1) and glycerin (2).
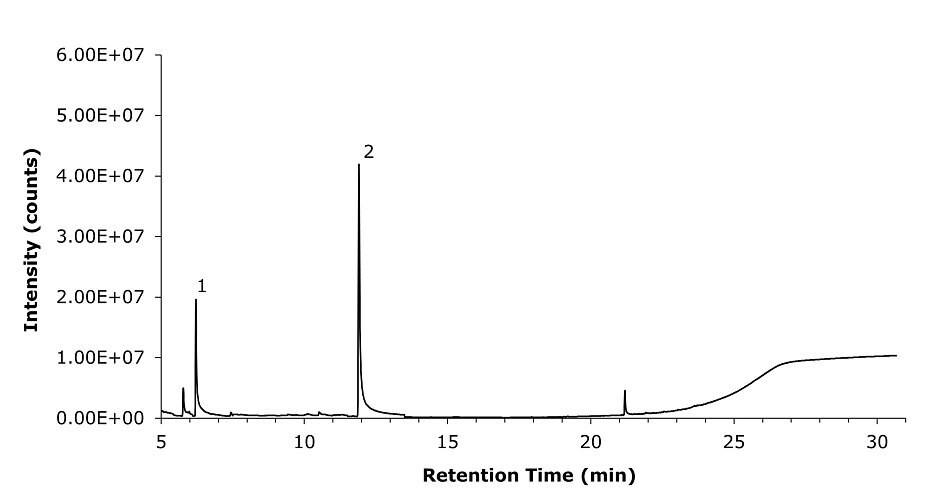
Figure 4.GC-MS chromatogram recorded for commercially available cough syrup sample B, prepared in diluent, showing the presence of propylene glycol (1), glycerin (2).
Conclusion
This study presents a GC-MS/MS method for quantifying ethylene glycol, propylene glycol, diethylene glycol, triethylene glycol, glycerin and sorbitol present in formulations for patients such as cough syrup. The method development utilized an Equity-1701 30m x 0.25 mm column with a film thickness of 0.25 µm. The % RSD for system repeatability with standard solution (SS) of the glycol mixture was well below 10% (3.63 to 8.89%). Derived from the external calibration data (R2≥0.962) the estimated potential LODs of glycols present in the original cough syrup samples for 5 compounds in the range of 2.78 mg/g to 3.74 mg/g, 50.12 mg/g for sorbitol), and the LOQs in the range of 8.41 mg/g to 11.33 mg/g, for sorbitol 126.5 mg/g. Analysis of the commercial sample A spiked with propylene glycol and glycerin showed recovery rates between 80.8% and 108 %. The second commercial sample B also spiked with propylene glycol and glycerin displayed recovery rates ranging from 73.6% to 112.8%. Sorbitol being more denser and possess higher boiling point as compared to other glycols, the development required higher concentration for sorbitol on GC-MS/MS technique, thus the studies of linearity and repeatability exhibits higher sorbitol concentrations.
Read more about Pharmeceutical Analysis & Quality Control
References
To continue reading please sign in or create an account.
Don't Have An Account?