Enhance API Solubility with Additive Manufacturing (3D Printing) and Mesoporous Silica in Pharmaceutical Formulation
Section Overview
- Advantages of Additive Manufacturing (3D Printing) Technologies in Pharmaceutical Formulation
- Additive Manufacturing (3D Printing) with Mesoporous Silica for Enhanced API Solubility
- Case Study 1: In-Blister Printing Using SoluPrint™ Additive Manufacturing with Parteck® SLC Mesoporous Silica
- Case Study 2: Formulation of a High-Dose 3D Printed Tablet
- Summary of Findings: Benefits of the Presented 3D Printing Platform Approach
- Conclusion
- Related Products
Additive manufacturing, or 3D printing, offers a transformative approach to the design and manufacture of solid dosage forms, enabling unprecedented flexibility through digital, layer-by-layer construction of tablets. When combined with solubility enhancement strategies, for example, by loading the active on the inorganic drug carrier Parteck® SLC mesoporous silica, this approach creates the opportunity to improve the formulation of poorly soluble active pharmaceutical ingredients (APIs).
In additive manufacturing, a homogeneous layer of silica is first deposited onto a print bed (Figure 1). A print head then applies the API fluid binder into predefined areas, allowing for fine control over layer deposition and binder application. As dedicated layers build up, the final product forms as silica tablets with uniformly distributed, amorphous API.
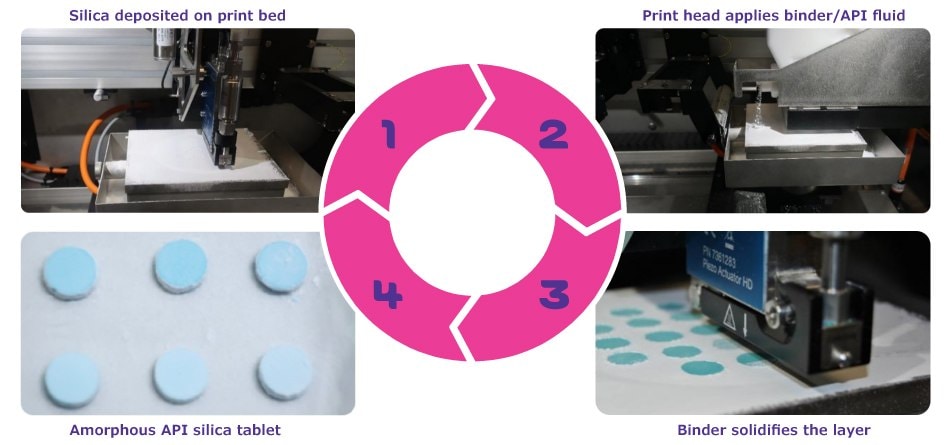
Figure 1.The SoluPrint™ 3D printing technology using Parteck® SLC drug carrier.
This page highlights the advantages of additive manufacturing for API formulation and provides case studies that describe the suitability of the approach to advance solid formulation development and address solubility challenges.
Manufacturing of the tablets for the case studies below was performed in collaboration with Aprecia Pharmaceuticals at the Aprecia facilities using their in-blister 3DP manufacturing platform. Aprecia is the leader in advanced additive manufacturing for the pharmaceutical industry that enables oral drug delivery through novel formulations and dosage forms.
Advantages of Additive Manufacturing (3D Printing) Technologies in Pharmaceutical Formulation
There are several advantages of additive manufacturing when used for the formulation of small molecule APIs:
- Personalization: Enables tailored drug formulations based on individual patient needs.
- Controlled Release Profiles: Allows for customization of drug release kinetics to optimize therapeutic outcomes.
- Accelerated Clinical Development: Simplifies development and improves responsiveness to emerging clinical demands.
- Sustainability: Reduces environmental footprint through streamlined development processes and efficient use of APIs.
- Decentralized Manufacturing: Enhances supply chain resilience by enabling local or on-demand production.
- High Flexibility and Smart Manufacturing: Supports adaptive, digitalized production with access to rich process data for continuous optimization and monitoring.
In addition to these benefits, the 3D printing process can be combined with solubility-enhancing excipients to overcome the solubility challenges presented by some APIs.
For alternative approaches to 3D printing, please read our related technical articles Solid Formulation Development Using Melt-based 3D printing Technologies and 3D Printed Precision: Selective Laser Sintering for Oral Drug Delivery.
Additive Manufacturing (3D Printing) with Mesoporous Silica for Enhanced API Solubility
An effective strategy for enhancing the solubility of poorly water-soluble APIs is a combination of Soluprint™ binder jetting 3D printing and Parteck® SLC mesoporous silica, which enables the conversion of crystalline drugs into more bioavailable amorphous forms. In binder jetting, a liquid binder is selectively deposited onto a powder bed to bind particles layer by layer, forming solid structures. This approach enables high flexibility in early prototyping, reduces development time, conserves valuable API resources, and facilitates the production of small, personalized batches of drug substance.
With 3D printing, the loading and tableting steps traditionally required in tablet production are combined, simplifying the overall process (Figure 2). This streamlined approach offers formulators greater flexibility by reducing both time and resource demands. Most importantly, it creates new possibilities for formulating challenging compounds that may have been difficult, or even impossible, to process using conventional methods.
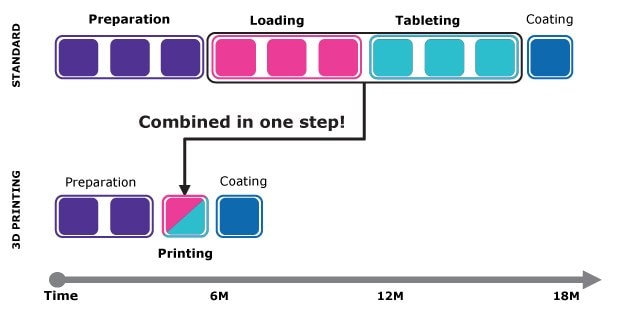
Figure 2.Coupling SoluPrint™ technology and Parteck® SLC mesoporous silica provides substantial time and resource savings for the formulation of challenging compounds.
Figure 3 shows two tablet production routes using the SoluPrint™ method with Parteck® SLC mesoporous silica. The open-bed printing concept (Figure 3A) combines the loading and 3D printing processes. In this approach, the drug and binder are individually distributed and layered sequentially, allowing precise control over composition and structure, layer by layer. The in-blister printing method (Figure 3B) uses preloaded Parteck® SLC mesoporous silica, with the loading step performed in advance. The 3D structure is then built by selectively applying the binder, enabling efficient and consistent tablet formation directly within the blister packaging.
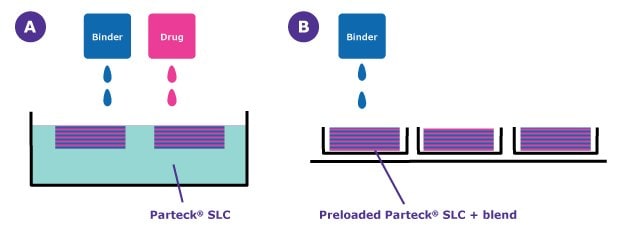
Figure 3.Comparison of A) open-bed and B) in-blister printing. In-blister printing uses API-loaded Parteck® SLC mesoporous silica in combination with an excipient blend and applies binder to form tablets directly in blister packaging.
For details on the working principle behind solubility enhancement with Parteck® SLC mesoporous silica, please read our dedicated technical article Improving API Solubility with Mesoporous Silica.
We invite you to watch our webinar, A new opportunity for challenging drug substances: Combining 3D printing technology with loading of mesoporous silica with additional expert insights on the topic.
Case Study 1: In-Blister Printing Using SoluPrint™ Additive Manufacturing with Parteck® SLC Mesoporous Silica
In this study, in-blister 3D printing was used to formulate 50 mg carbamazepine tablets. Carbamazepine (CBZ), a BCS class II compound known for its poor solubility, was loaded onto the Parteck® SLC mesoporous silica and combined with a binder liquid and a blend of other excipients to produce the final tablet.
Uniformity of the Formulation
To assess the uniformity of the formulation, scanning electron microscopy-energy dispersive X-ray (SEM-EDX) imaging was performed on tablet cross-sections. The results confirmed a highly homogeneous distribution of the silica particles, an essential early step in demonstrating both the feasibility and reproducibility of the process (Figure 4).
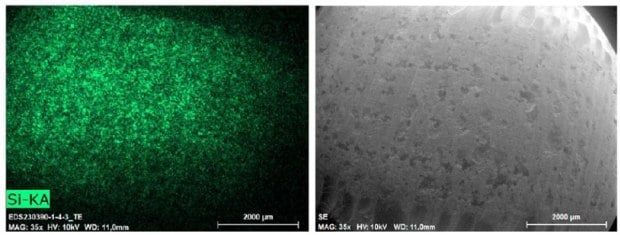
Figure 4.SEM-EDX imaging of tablet cross-sections.
Characterization of the API
Powder X-ray diffraction (PXRD) confirmed the successful transformation of carbamazepine into its amorphous form due to the absence of distinct crystalline carbamazepine peaks in the final printed formulation (Figure 5A). Dissolution studies demonstrated a rapid onset of drug release and sustained supersaturation when compared to the crystalline form (Figure 5B). These findings demonstrated the potential of in-blister 3D printing to enhance the bioavailability of poorly soluble compounds.
A

B
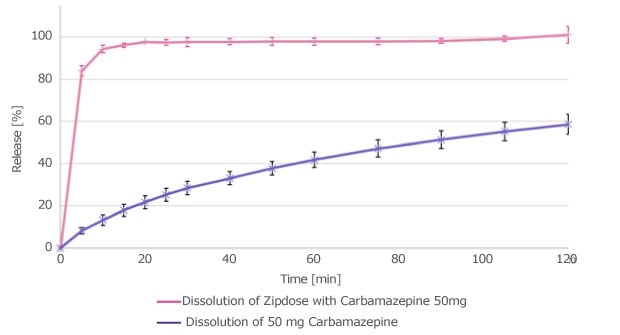
Figure 5. A) Solid state and B) dissolution profile of the 3D printed carbamazepine tablet compared to the crystalline API and, in the case of PXRD results, a placebo 3D printed tablet.
Case Study 2: Formulation of a High-Dose 3D Printed Tablet
In this case study, a 200 mg carbamazepine tablet production process included adding and leveling a layer of preloaded Parteck® SLC mesoporous silica directly into the blister cavity, followed by repeated binder printing to build the tablet structure (Figure 6). In order to maximize API load and simplify the formulation in this case study, other excipients were omitted. The printed tablet was then tamped, smoothed, dried, and ejected, resulting in a homogeneous, visually appealing product formed directly in its final packaging.
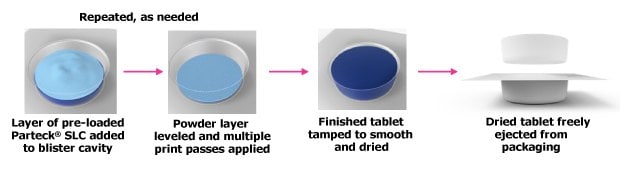
Figure 6.In-blister, 3D tablet printing process.
Tablet Characterization
Tablet characterization data is provided in Table 1, including dose per tablet, total mass achieved, and drug content expressed as a percentage. The results show high repeatability, with low standard deviations, particularly in drug content, demonstrating the precision and consistency of the process. Raman mapping was used to demonstrate that the drug was homogeneously distributed throughout the tablet (data not shown).
Dissolution Performance
Dissolution performance of the tablets from both case studies was evaluated by comparing different tablet formulations. Varying the printing conditions allows for fine-tuning of the formulation to influence drug release. In this case, a standard carbamazepine formulation was compared to the two tablet formulations, one with a 50 mg load and another with a high 200 mg load. The 50 mg tablet showed a rapid onset of dissolution, while the 200 mg tablet, composed mostly of the active ingredient, also demonstrated strong dissolution performance over time (Figure 7). This highlights the platform’s potential for controlled release applications, tailoring the drug release profile according to the individual need.
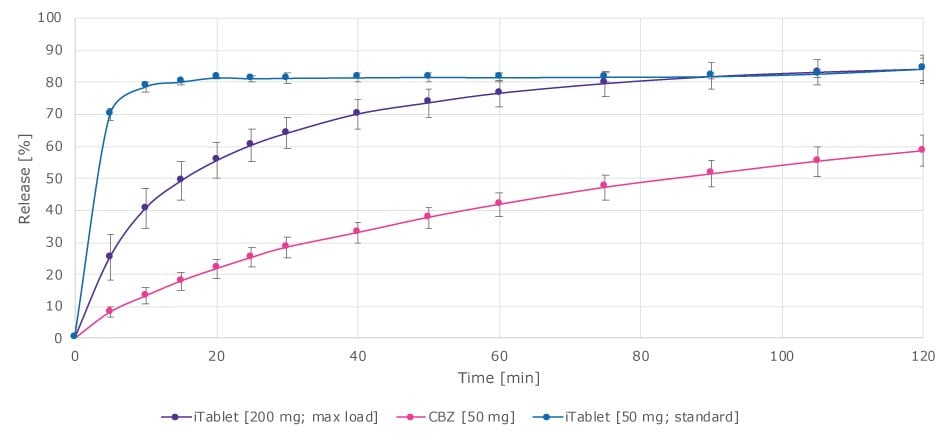
Figure 7.Dissolution performance of tablet formulations from the two case studies.
Summary of Findings: Benefits of the Presented 3D Printing Platform Approach
The two case studies described above demonstrate the flexibility of the 3D printing platform using pre-loaded carbamazepine in Parteck® SLC mesoporous silica. Both used the same custom binder print fluid, but with different formulation strategies (Table 2).
Study 1 included additional excipients, such as mannitol, povidone, and flavor components, blended with the drug-loaded mesoporous silica. These excipients contributed to binding and tablet properties, allowing for a well-formed, aesthetically pleasing dosage form. However, drug loading was lower (5%) due to the presence of other ingredients.
Study 2 focused on a simplified formulation using only the drug-loaded silica and binder fluid, without added excipients. Despite requiring a higher binder percentage to achieve comparable hardness, this approach enabled a much higher drug loading (18.1%) while maintaining rapid disintegration and tablet quality.
These examples highlight how the binder fluid and printing process can be adapted to different formulation needs, whether optimizing for excipient compatibility or maximizing drug content, and that even a simplified formulation yields excellent results.
Conclusion
Additive manufacturing (3D printing) is redefining how solid dosage forms are developed, introducing a digital, layer-by-layer method that allows for precise control over tablet design. When combined with techniques to improve drug solubility, such as Parteck® SLC mesoporous silica, this approach holds significant promise for advancing the formulation of otherwise difficult-to-deliver APIs in this type of manufacturing technology.
Looking ahead, the evolution toward cavity or in-blister printing adds another layer of adaptability, particularly in streamlining manufacturing and accelerating product development. The ability to combine drug loading directly with printing reduces the number of processing steps, creating a faster, more efficient development path.
Importantly, the successful transfer of the SoluPrint™ technology from lab-scale experiments to Aprecia Pharmaceutical’s cGMP-capable in-blister 3DP manufacturing platform demonstrates not only technical feasibility but also scalability, supporting both early-stage clinical supply and the potential for commercial batch production. This evolution underscores the promise of 3D printing as a versatile, scalable, and future-ready technology for pharmaceutical manufacturing.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?