N-Linked Glycans Overview
N-linked glycosylation Introduction
N-linked glycosylation, modification, and degradation are involved in a wide variety of processes in all organisms from archaea to eukaryotes. It is the most common covalent protein modification in eukaryotic cells. No other post-translational protein modification is as chemically complex or serves as many diverse functions.
Structures
All N-linked glycans are based on the common core pentasaccharide, Man3GlcNAc2 (Figure 1). Further processing in the golgi results in three main classes of N-linked glycan classes:
- High-mannose
- Hybrid
- Complex
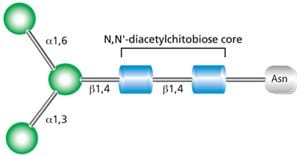
Figure 1. Core structure for all N-linked glycans.
High-mannose glycans contain unsubstituted terminal mannose sugars (Figure 2). These glycans typically contain between five and nine mannose residues attached to the chitobiose (GlcNAc2) core. The name abbreviations are indicative of the total number of mannose residues in the structure.
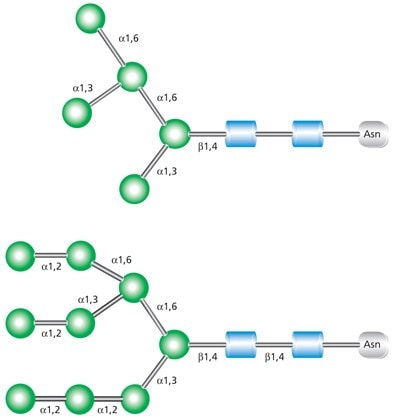
Figure 2. Examples of high mannose glycans Man-5 (top) and Man-9 (bottom).
Hybrid glycans are characterized as containing both unsubstituted terminal mannose residues (as are present in high-mannose glycans) and substituted mannose residues with an N-acetylglucosamine linkage (as are present in complex glycans) (Figure 3). These GlcNAc sequences added to the N-linked glycan core in hybrid and complex N-glycans are called “antennae”. The uppermost structure shown in Figure 3 is a biantennary glycan with two GlcNAc branches linked to the core. The lower structure is a triantennary glycan with three GlcNAc branches.
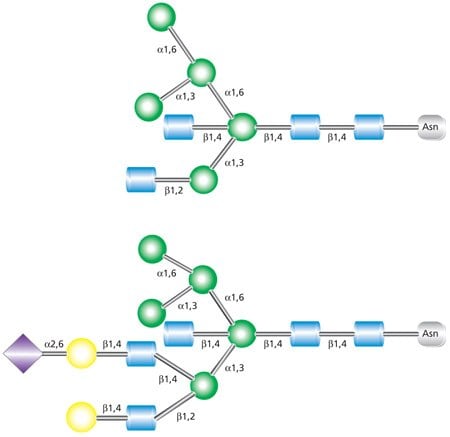
Figure 3. Examples of biantennary (top) and triantennary (bottom) hybrid glycans.
Complex N-linked glycans differ from the high-mannose and hybrid glycans by having added GlcNAc residues at both the α-3 and α-6 mannose sites (Figure 4). Unlike the high-mannose glycans, complex glycans do not contain mannose residues apart from the core structure. Additional monosaccharides may occur in repeating lactosamine (GlcNAc-β(1→4)Gal) units. Complex glycans exist in bi-, tri- and tetraantennary forms and make up the majority of cell surface and secreted N-glycans. Complex glycans commonly terminate with sialic acid residues. Additional modifications such as the addition of a bisecting GlcNAc at the mannosyl core (as indicated in Figure 4) and/or a fucosyl residue on the innermost GlcNAc are also possible.

Figure 4. Example of tetraantennary complex glycan that contains terminal sialic acid residues, a bisecting GlcNAc on the pentasaccharide core, and fucosylation on the core GlcNAc.
Biosynthesis and Degradation
Protein glycosylation of N-linked glycans is actually a co-translational event, occurring during protein synthesis. N-linked glycosylation occurs at the consensus sequence Asn-X-Ser/Thr, where the glycan attaches to the amine group of asparagine and X represents any amino acid except proline. Glycosylation occurs most often when this consensus sequence occurs in a loop within the peptide.
Oligosaccharide intermediates destined for protein incorporation are synthesized by a series of transferases on the cytoplasmic side of the endoplasmic recticulum (ER) while linked to a dolichol phosphate (P-Dol) base. Following the addition of mannose and N-acetyl-D-glucosamine molecules, typically using GDP-Man and UDP-GlcNAc as glycan donors, the final construct prior to movement from the cytoplasmic side of the ER is Man5GlcNAc2-P-Dol (Figure 5).
This dolichol precursor with its attached glycan is shifted to the lumen of the ER (“flipped”) for further enzymatic modification. Processing includes trimming of glucose and mannose residues by glycosidases and addition of new residues via glycosyltransferases in the ER and, to a great extent, in the Golgi. In the Golgi, high-mannose N-glycans can be converted to a variety of complex and hybrid forms that are unique to vertebrates. The completed oligosaccharide is then transferred from the dolichol precursor to the Asn of the target protein by oligosaccharyltransferase (OST).
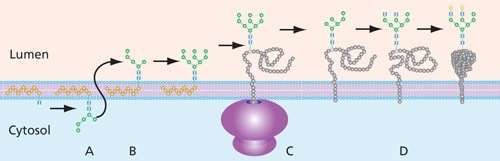
Figure 5. General process of N-linked glycan construction in the ER. The glycan precursor is assembled on a dolichol phosphate base while in the cytoplasm of the ER (A). Once the glycan is switched to the lumen side (B), the glycan has additional sugars added and removed (trimmed) before being attached to the protein (C), where additional processing takes place (D).
Cleaved high-mannose glycans serve as substrates in the golgi where additional modification and diversification occurs. High-mannose structures are trimmed by the action of mannosidases, removing the mannose extensions and making the glycan available for conversion to hybrid and complex glycans by subsequent addition of GlcNAc sugars (“antennae”) by the actions of N-acetylglucosyl transferases. The mammalian gene Mgat1 codes for the enzyme N-acetylglucosyl transferase-I (GlcNAcT-I), which is responsible for the addition of one GlcNAc in the β(1→2) linkage. The subsequently modified glycan becomes a substrate for α-mannosidase II which removes the α(1→3) and α(1→6) mannose residues and results in a molecular structure that is subject to glucosyl group donation by GlcNAcT-II (N-acetylglucosyl transferase-II), encoded by the Mgat2 gene. Catalysis by GlcNAcT-II converts the hybrid glycans to the complex forms by attaching additional GlcNAc moieties to the hybrid structure.
Fucosylation at the core GlcNAc residue following GlcNAcT-I modification in both hybrid and complex N-linked synthesis is also a common occurrence. In vertebrates, fucose is added in an α(1→6) linkage, while in plants and invertebrates, fucose is added in an α(1→3) linkage.
Inhibition or elimination of glycosylation in the study of N-linked glycans can be brought about by a number of compounds. N-glycosylation is strongly inhibited in the presence of compactin, coenzyme Q, or exogenous cholesterol. Treatment with tunicamycin completely blocks glycosylation as tunicamycin inhibits GlcNAc C-1‑phosphotransferase, an enzyme that is critical in the formation of the dolichol precursor.
α-Mannosidase, β-mannosidase, sialidase and α-fucosidase are the primary exoglycosidases involved in N-linked glycan trimming and degradation. Insect cells express an N-acetylglucosminidase that cleaves terminal GlcNAc residues from N-linked glycans.
Functions
During development, the intermediate structures of a glycoprotein perform specific functions. In the early phases of glycoprotein evolution, different core oligosaccharide structures are necessary for proper protein folding and functional group orientation. Improperly folded proteins are either reglycosylated and refolded, or deglycosylated and degraded. In later phases, the oligosaccharide moiety is required for intracellular transport and targeting of the glycoprotein in the endoplasmatic reticulum, Golgi complex, and trans-Golgi network. In the final phase, the N-linked glycan undergoes extensive modification in the Golgi complex, resulting in a mature glycoprotein.
The protein moiety and the attached hydrophilic N-linked glycans are relatively independent, despite being covalently linked, i.e. glycans can be modified without significantly affecting the protein structure and function. Proteins may contain multiple glycosylation sites that are modified with any of the three classes of N-linked glycans. Vertebrates have been found to possess a diverse compliment of complex and hybrid glycoproteins due to the broad variety of glycosidases and glycosyltransferases coded within the genome. While these three classes of Nlinked glycoproteins are also present in lower organisms, there is less diversity of structure than is found in vertebrate glycoproteins.
In addition to a specific glycoprotein being able to contain multiple glycan structures, different molecules of the same glycoprotein may have different glycan structures attached to the identical substitution site. Modifications in the glycan structures provide identity characteristics to different cell types and to the same cell type at stages of development, differentiation, transformation, maintenance, and aging. This variation in protein glycosylation is known as microheterogeneity and contributes to the difficulty in identifying and isolating specific glycoproteins.
The congenital disorders of glycosylation (CDG) are a series of diseases associated with errors of metabolism due to enzyme deficiencies. The majority of identified CDGs are due to failures in the biosynthesis or degradation of N-glycans because of absence of one of the enzymes involved in N-glycosylation, primarily the exoglycosidic enzymes α-mannosidase, β-mannosidase, sialidase, or α-fucosidase.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?