Three-Dimensional Bioprinting for Tissue and Disease Modeling
Introduction
Three-dimensional (3D) bioprinting is developing rapidly, and offers enormous potential for the fabrication of living tissue constructs.1,2 In the most common form, bioprinting uses a computer-assisted motorized device for the layerby-layer deposition of biocompatible materials, viable cells, growth factors, proteins, nucleic acids, drugs, and supporting components into precise geometries to create functionally and structurally biomimetic tissue constructs.
In recent years, 3D bioprinting has significantly advanced the field of tissue and disease modeling to facilitate drug development and therapeutic screening. In general, bioprinting is based on one of a few main technological approaches such as: extrusion, inkjet, laser-assisted, or steriolithographic.3 Extrusionbased bioprinting utilizes mechanical or pneumatic forces to dispense a bioink through a nozzle to form a continuous filament. Currently the most commonly used bioprinting method, extrusion-based bioprinting is comparatively slow and has lower resolution, but can fabricate constructs from highviscosity bioinks with reasonable cell viability. Inkjet bioprinting employs thermal, piezo, or acoustic forces to deposit a bioink in the form of droplets, offering fast fabrication speeds but low cell densities. While extrusion bioprinting can typically work with high viscosity bioinks, inkjet bioprinting requires bioinks with low viscosities. Laser-assisted bioprinting is a nozzle-free technique that uses laser pulses to deposit bioink from the donor slide onto the receiver substrate. Although this allows deposition of highly viscous and densely cellularized bioinks, it is limited by the lower cell survival rate. Finally, stereolithographic bioprinting utilizes precisely controlled patterns of light to photopolymerize photosensitive polymers on a vertically movable collecting platform in a layer-by-layer process, thus forming 3D constructs of the desired structure. Stereolithographic bioprinting offers higher resolution, rapid fabrication, and higher cell viability than other methods.
Despite the different approaches, most bioprinting processes consist of a similar series of steps: (i) construction of the 3D model using computer-aided design software, (ii) development/selection of the bioink, usually a combination of one or more compatible biomaterials and viable cells, depending on the modality and specific tissue to be bioprinted, (iii) robotically programmed bioprinting with in-printing and/or post-printing physical and/or chemical crosslinking, and (iv) maturation of the bioprinted tissue construct. Generally, the matured bioprinted tissue constructs are specifically designed for applications in tissue engineering and regenerative medicine.2 More recently, the fabrication of functional tissue models using 3D bioprinting approaches have been used in disease modeling, drug development, and screening of personalized therapeutics.4 Herein we briefly review bioink selections and provide representative examples relating to the applications of 3D bioprinting in tissue model biofabrication (Figure 1).
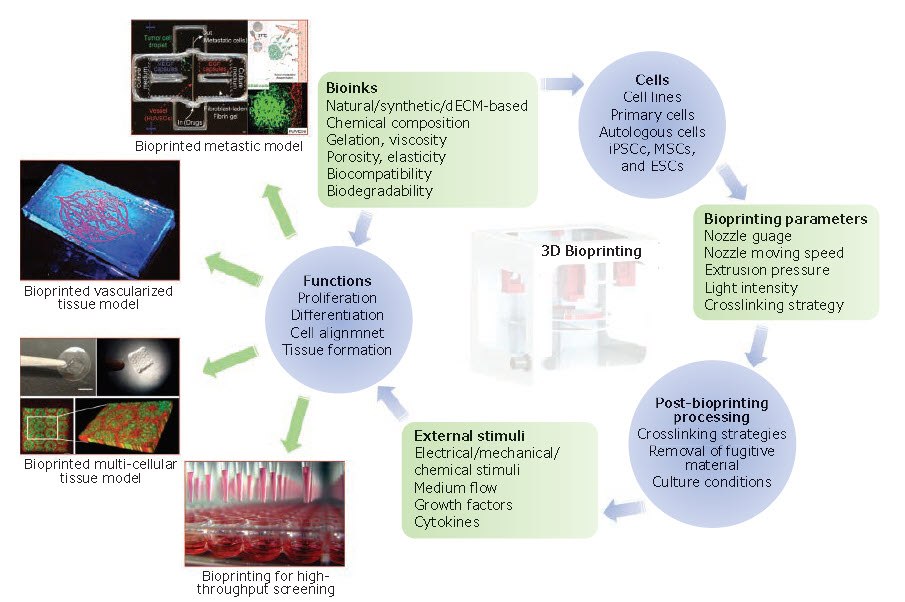
Figure 1. The essential components and applications of 3D bioprinting. Bioinks, bioprinting parameters, and post-bioprinting processing all impact viability and functionality of cells, which in turn affect subsequent cellular events, such as proliferation, differentiation, and tissue formation. hiPSCs: Human induced pluripotent stem cells, MSCs: Mesenchymal stem cells and ESCs: Embryonic stem cells.18, 27, 28
Bioink development and choice of biomaterials
Bioinks are one of the most important components of 3D bioprinting. A bioink is essentially a hydrogel biomaterial, containing one or more cell types, nutrients, and growth factors in varying amounts, that mimics the extracellular matrix (ECM) of the tissue and supports the growth of the embedded cells.5 Designing biologically relevant bioinks is one of the critical challenges for fabrication of 3D-bioprinted tissue constructs. The bioink should not only provide structural, physical, and mechanical support to the embedded cells, but also supply them with the essential biological and chemical cues for cell survival, proliferation, and differentiation required for tissue morphogenesis and homeostasis. The selection of proper biomaterial (or combination of biomaterials) for a bioink is a key step for successful bioprinting of tissues, and it depends on several factors such as the bioprinting modality to be used, the tissue of interest, and any post-printing processes that are required. In general, an ideal bioink should (i) have good printability; (ii) be curable with a cell-friendly method, (iii) have suitable mechanical strength to maintain the structural integrity of tissue of interest, (iv) be biocompatible and biodegradable without eliciting any toxicity or immunological reactions, (v) mimic the in vivo microenvironment to support and promote cellular activities such as adhesion, migration, proliferation, and differentiation of living cells (Figure 2A–B).
To date, numerous hydrogel biomaterials have been used for the formulation of bioinks for 3D bioprinting of tissues (Figure 2C). These include, for example, naturally-derived biomaterials based on collagen, gelatin, alginate, fibrin, hyaluronic acid, silk proteins, chitosan, and decellularized ECM (dECM), as well as synthetic biomaterials such as poly(ethylene glycol) (PEG), poly(hydroxyethyl methacrylate), and polyvinyl alcohol.4 Natural sources are a favorable subset of biomaterials because of their biocompatibility, tunable degradation, and intrinsic resemblance to native ECM. However, weak mechanical strength and inconsistency in compositions and properties between production batches are common drawbacks.6 On the other hand, synthetic biomaterials are highly defined and reproducible with tunable compositions. In addition, they are often biologically inert, being both nontoxic and nonimmunogenic. The mechanical properties, degradation rate, and composition of synthetic biomaterials can be easily controlled. However, synthetic materials often lack adequate sites for cell adhesion and do not exhibit the complexity of native ECM.7 Therefore, synthetic materials are often combined with natural biomaterials to overcome these limitations, and to engineer tissue-like microenvironments that mimic both the chemical and physical characteristics of native ECM. For example, 4-arm PEG-tetraacrylate (PEGTA)-gelatin methacryloyl (GelMA) and 8-arm PEG-octoacrylate (PETOA)-GelMA composite bioinks were developed for 3D bioprinting of biomimetic vascular tissues.8,9 The addition of PEG derivatives to GelMA provides adequate rheological and other mechanical properties that facilitate the bioprinting of complex multilayer hollow structures.
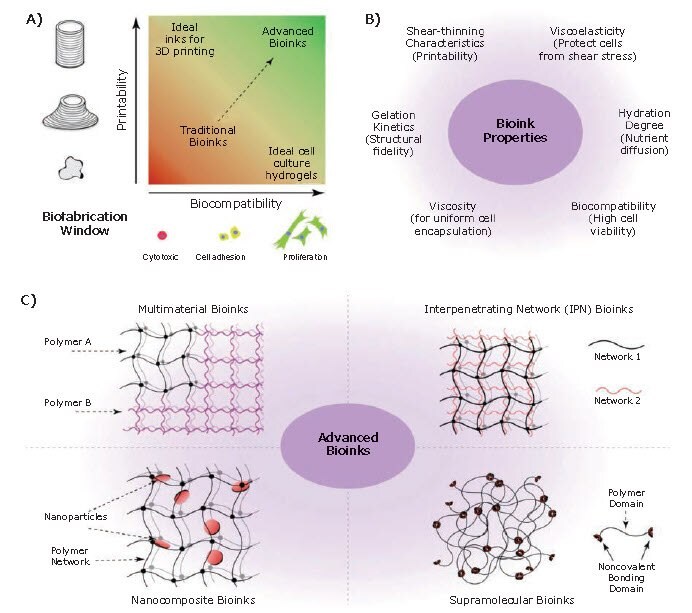
Figure 2. Bioinks for 3D bioprinting. A) Rational approach for designing bioinks requires considering both printability and biocompatibility. B) Properties of an ideal bioink. C) Classification of advanced bioinks into four major groups.5
However, these composite hydrogels still may not possess sufficient biomimetic physicochemical properties to deliver tissue-specific functions. As a result, dECM-derived hydrogels have been increasingly used as bioinks. The decellularization process uses a combination of mechanical, chemical, and enzymatic treatments to remove all cellular components, yielding a collagenous matrix that keeps many of the structural ECM components and soluble factors intact. Studies have shown that dECM preserves the biochemical composition of the native ECM tissue from which it is derived, which proves important for maintaining phenotypes and functionality of the cells when forming biologically relevant tissues. For example, bioprinted renal constructs using kidney dECM-derived bioink exhibited physiologically relevant features of the native renal tissue.10 Similarly, 3D bioprinting of the dECM-based bioinks derived from pepsin-solubilized heart, adipose, and cartilage tissues exhibited high cell viability and functionality of the bioprinted rat myoblasts, human adipose-derived stem cells, and human mesenchymal stem cells in the respective tissue constructs.11
Bioprinted tissue/disease models and their applications in probing drug effects
Bioprinted tissue constructs with spatially controlled architectures represent important in vitro tools for tissue and disease modeling, with relevant applications in toxicology. These models allow study of the biochemical, genetic, and histological consequences of specific drugs thus providing pharmacokinetic, pharmacodynamic, and toxicity information. In this section, we examine some applications of 3D-bioprinted tissue and disease models in studying tissue-specific functions, drug-metabolizing activities, and drug responses. The representative bioprinted tissue models used for drug screening applications are listed in Table 1.
Cardiac tissue models
Bioprinting has the potential to generate physiologically relevant, 3D contractile cardiac tissues for drug testing applications. For example, rat primary cardiomyocytes encapsulated in a fibrinbased bioink containing fibrinogen, gelatin, aprotinin, glycerol, and hyaluronic acid were bioprinted to create cardiac tissue constructs with spontaneous and synchronous contractions in in vitro culture.12 These constructs were evaluated for physiological responses to known cardiotoxic drugs including epinephrine and carbachol. Epinephrine (200 nM) increased the beating frequency from 80 to 110 beats per minute, while carbachol (10 μM) was found to decrease the beating frequency to 40 beats per minute. As blood vessels play an important role in transporting nutrients, oxygen, and drugs in and out of tissues including the heart, another recent example demonstrated fabrication of 3D endothelialized microfibrous scaffolds by extrusion bioprinting of human umbilical vein endothelial cells (HUVECs)-loaded alginate/GelMA blend bioink (Figure 3A–B). This was further seeded with neonatal rat cardiomyocytes or iPSC-derived cardiomyocytes, thus generating endothelialized myocardial tissues capable of spontaneous and synchronous contractions (Figure 3C).13 This tissue model was later integrated in a microfluidic bioreactor and used to study the toxicity of an anti-cancer drug, doxorubicin, in a dose- and time-dependent manner. When exposed to doxorubicin for 6 days, the beating rate of cardiomyocytes decreased with simultaneous reduction in the secretion of von Willebrand factor (vWF) by the HUVECs (Figure 3D–E).
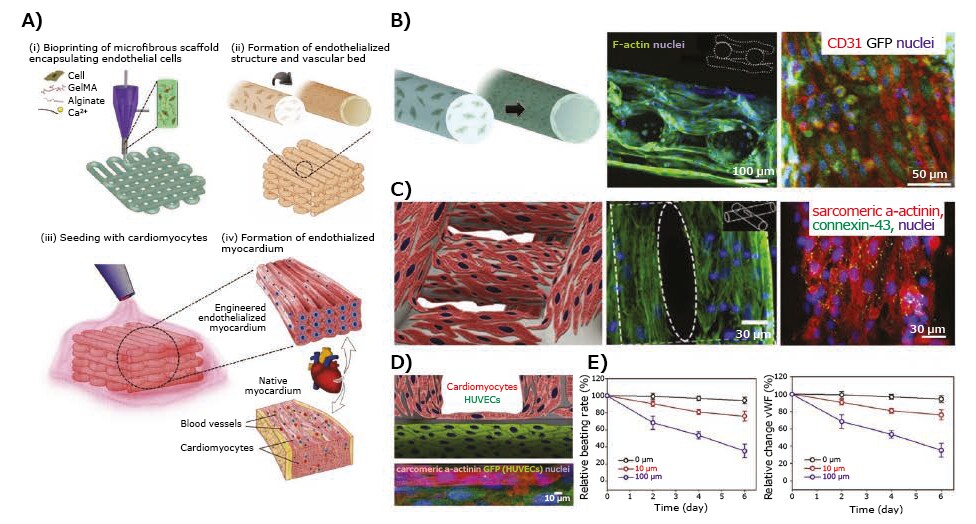
Figure 3.Application of 3D-bioprinted tissue models in drug testing — 3D-bioprinted endothelialized myocardium model. A) Schematics showing the procedure of fabricating endothelialized myocardium tissue model. B) Schematic showing the assembly of HUVECs in the bioprinted microfibers into a confluent layer of endothelium on the peripheries, as well as confocal fluorescence images showing the cross-sectional view of a three-layer scaffold, and tight junction formation between the HUVECs. C) Schematic showing a scaffold seeded with neonatal rat cardiomyocytes, F-actin staining showing the distribution of cardiomyocytes on the surface of the microfibers, and immunofluorescence staining of sarcomeric α-actinin and connexin-43 expressions. D) Schematic and high-resolution confocal fluorescence micrograph showing an endothelialized myocardial tissue. E) Relative beating of the endothelialized myocardial tissues and the levels of vWF expression by the endothelial cells, upon treatment with different dosages of doxorubicin.13
Kidney Tissue Models
Human kidneys filter approximately 180 L of plasma each day, reabsorbing water and solutes through renal tubules, and removing waste from the blood,14 making them susceptible to damage by drugs and toxins. Bioprinting is a promising method for fabricating kidney tissues or components (such as renal tubules) that exhibit renal filtration, reabsorption, and secretory functions. Homan et al. bioprinted renal proximal tubules using extrusion bioprinting, in which a sacrificial bioink made of Pluronic F127 and thrombin was first printed on a gelatin-fibrinogen-transglutaminase ECM. This was followed by removal of the Pluronic F127, creating a hollow tubule within the crosslinked ECM (Figure 4A–B).15 Proximal tubule epithelial cells were then seeded within the tubule and allowed to grow to maturity under continuous medium perfusion through the lumen (Figure 4C). The perfusable proximal tubules exhibited an epithelial-like morphology, which was disrupted when treated with cyclosporine A in a dose-dependent manner (Figure 4D). The same group later reported16 bioprinting of a 3D vascularized proximal tubule model, composed of two adjacent proximal tubule and vascular conduits embedded in an ECM, using their original ECM and fugitive bioink with slight modifications (Figure 4E). The proximal and vascular channels were seeded with proximal tubule epithelial cells and glomerular microvascular endothelial cells, respectively (Figure 4F). Biomacromolecule uptake was studied using fluorescently labeled albumin and inulin, and albumin was found to be reabsorbed selectively. Further, epithelium-endothelium crosstalk was studied by circulating high-glucose (400 mg glucose/dL) medium with or without dapagliflozin and normal glucose (100 mg glucose/dL) medium through the proximal tubule and monitoring both glucose reabsorption and endothelial cell dysfunction.
Liver Tissue Models
Hepatotoxicity remains the primary reason for late-stage failures of many drugs,17 making liver drug toxicity studies crucial for drug development. To this end, 3D bioprinting has been used to create liver tissue models that reliably reproduce drugs metabolism, as well as glucose and lipid metabolisms. An example of this is when digital light processing-based stereolithographic bioprinting was employed to develop a 3D hepatic lobule model with patterned human induced pluripotent stem cell (hiPSC)-derived hepatic cells, HUVECs, and the adipose-derived stem cells in a physiologically-relevant architecture using GelMA and glycidal methacrylate-hyaluronic acid.18 The authors investigated the expression of different hepatic marker genes and enzymes involved in drug metabolism in hiPSC-derived hepatic progenitor cells (hiPSC-HPCs) in the bioprinted 3D hepatic model, and found that among the five cytochromes P450 (CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4), the expressions of the most common CYP, CYP3A4, were significantly higher in hiPSC-HPCs. Approximately half of the drugs used today are estimated to be metabolized by CYP3A4,18 making this a promising model system. They further evaluated the effect of rifampicin, an antibiotic with potential hepatotoxicity, and showed that rifampicin significantly increased the expressions of CYP3A4, CYP2C9, and CYP2C19 in hiPSCHPCs in the 3D-bioprinted hepatic model when compared to untreated controls. In another study by Nguyen et al.,19 a liver tissue model was fabricated by the microextrusion method using primary human parenchymal cells (100% hepatocyte cellular paste, generated via compaction) and non-parenchymal cells (HUVECs and hepatic stellate cells in NovoGel hydrogel).Their responses to the known hepatotoxicant trovafloxicin were studied in comparison to levofloxacin, revealing that trovafloxacin induced significant toxicity at clinically relevant doses (≤ 4 μM) in a dose-dependent manner. Similarly, an extrusion-bioprinted liver model using HepG2 cell-laden Matrigel was used in a microfluidic system to analyze the metabolism of amifostine, an anti-radiation prodrug.20 Moreover, 3D bioprinting of human HepG2/C3A spheroids was achieved within the GelMA bioink directly in a bioreactor (Figure 5A–B).21 The liver-on-a-chip platforms with bioprinted hepatic spheroids were cultured under medium perfusion and analyzed for albumin, alpha-1 antitrypsin (A1AT), transferrin, and ceruloplasmin secretions as well as for expressions of cytokeratin 18, multi-drug resistance-associated protein 2 (MRP-2), and tight junction protein ZO-1 in hepatocytes within the bioprinted constructs (Figure 5C). The toxicity of acetaminophen (APAP) was further evaluated (Figure 5D), demonstrating application of the model for drug toxicity testing.
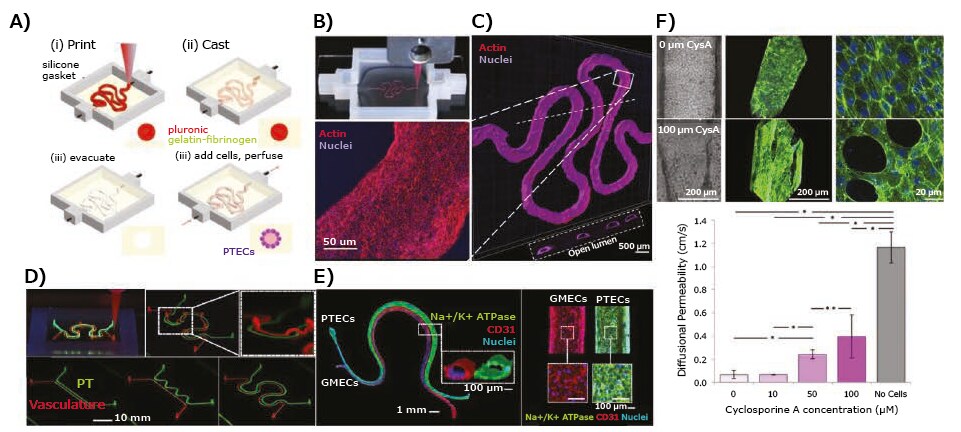
Figure 4. Application of 3D-bioprinted tissue models in drug testing — 3D-bioprinted convoluted renal proximal tubule model. A) Schematics of different steps in the fabrication of 3D renal proximal tubule. B) Photograph showing the bioprinting process of a proximal tubule (Pluronic F127 fugitive template). C) Confocal projection and 3D rendering images of the bioprinted convoluted proximal tubule populated with a confluent layer of proximal tubule epithelial cells. D) Cyclosporine A-induced disruption of the epithelial barrier function by quantifying the diffusional permeability of fluorescein isothiocyanate (FITC)-dextran (70 kDa). E) Simple and complex bioprinted vascularized proximal tubule (3D VasPT) models. F) Confocal images of the 3D VasPT containing epithelial cells in the proximal tubule and endothelial cells in the vessel. Reproduced under the Creative Commons Attribution License 4.0.15,16
Intestinal tissue models
The intestine is one of the main organs in which drugs are readsorbed. Recently, a bi-layered intestinal tissue model was generated by extrusion bioprinting of an interstitial layer using adult human intestinal myofibroblasts, and an epithelial layer using adult human intestinal epithelial cells suspended in a thermo-responsive NovoGel.22 The intestinal tissue constructs demonstrated a polarized epithelium with the expression of tight junction proteins such as E-cadherin, ZO-1, and functional CYP450 enzymes. Permeability studies were performed using Lucifer yellow, mitoxantrone, digoxin, propranolol, and topotecan. In addition, the toxicity of indomethacin, a nonsteroidal anti-inflammatory drug, was studied, and it was found that barrier function decreased in the bioprinted intestinal tissue in a dose-dependent manner.
Tumor models
In addition to normal tissues, bioprinting has shown promise in the fabrication of tumor tissue models that better replicate the native tumor microenvironment, which is critical for tumor cell proliferation, metastatic dissemination, and responses to pharmaceutical agents. An extrusion-based 3D bioprinted glioma model was established using a glioma stem cell-laden porous alginate/gelatin/fibrinogen bioink.23 The drug-sensitivity studies using this glioma model showed increased resistance to temozolomide when compared to monolayer cultures at concentrations of 400–1600 μg mL-1. More recently, a two-step extrusion bioprinting of mini-brains was reported in which the larger brain tissue was first bioprinted with an empty cavity using mouse macrophage cells, then the cavity was filled with mouse glioblastoma cells, with both cells encapsulated in the GelMA/gelatin blend bioink.24 The glioblastoma cells actively recruited macrophages, polarizing them into a glioblastomaassociated macrophage-specific phenotype. The effects of carmustine, a common chemotherapy for glioblastoma, and two immunomodulatory drugs, AS1517499 (a Stat6 inhibitor) and BLZ945 (an inhibitor of colony stimulating factor 1 receptor (Csf-1r)) were investigated. Moreover, a 3D cervical cancer tumor model was bioprinted using HeLa cells encapsulated in gelatin/alginate/fibrinogen, which showed increased chemoresistance to paclitaxel when compared to planar cultures.25 In another study, a 3D ovarian cancer model was generated by microvalve cell deposition (i.e., inkjet bioprinting) using OVCAR-5 human ovarian cancer cells and MRC-5 fibroblasts micropatterned on Matrigel.26 OVCAR-5 and MRC-5 cells were ejected simultaneously using dual ejector heads in a spatially controlled microenvironment, in a high-throughput and reproducible manner. OVCAR-5 cells overlaid on Matrigel spontaneously formed multicellular acini of ~100–500 μm2 in size and gradually increased heterogenicity over the 15-day culture period.
In addition, Meng et al.27 reported a 3D-bioprinted vascularized tumor model that mimics metastatic dissemination by integrating lung tumor cells (A549 cells), HUVECs-lined vascular conduits, and biochemical signals from 3D-bioprinted core/shell capsules. Growth factor-loaded GelMA hydrogel was chosen as the core, and gold nanorods-functionalized poly(lactic-co-glycolic acid) (PLGA) films as shells, while a fibroblast-laden, fibrin hydrogel matrix served as the main component of the tumor stroma. Tumor cell invasion into the surrounding matrix and intravasation into the vasculature were studied using epidermal growth factor and vascular endothelial growth factor gradients, which were dynamically released from the capsules. In addition, potency and targeting of two immunotoxin ligand-directed toxins, EGF4KDEL and CD22KDEL, were studied.
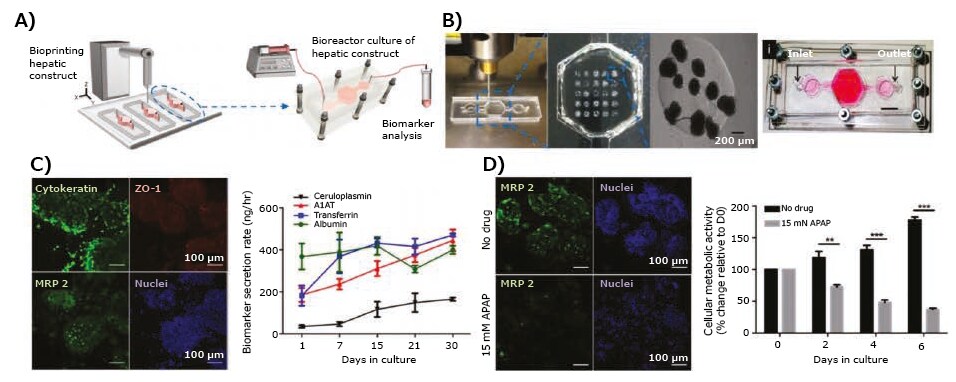
Figure 5. 3D-bioprinted liver model. A) Schematic of the hepatic bioreactor culture platform integrated with a bioprinter and biomarker analysis module. B) Bioprinting of GelMA hydrogel-based hepatic constructs directly within the bioreactor as a dot array, and topview of the assembled bioreactor (scale bar: 1 mm). C) Confocal images showing the bioprinted HepG2/C3A spheroids stained for cytokeratin 18, ZO-1, MRP-2, and nuclei, and the rate of secretion of hepatic biomarkers, albumin, A1AT, and ceruloplasmin, by HepG2/C3A spheroids. D) Confocal images showing MRP2 expressions and measured metabolic activity of the hepatic spheroids subjecting to APAP-induced hepatoxicity in a dose-dependent manner.21
Conclusions
The use of 3D-bioprinted tissue models for in vitro drug testing advanced significantly in recent years, and these tissue models show promise for enhanced reproducibility, which will reduce the cost of drug discovery and development through automated bioprinting operations. Another benefit lies in the potential of these bioprinted tissue models to reduce the use of animals for drug testing by both academic labs and pharmaceutical companies. Still, many challenges remain, such as the need for increased speed and resolution, availability of tissue and/or patient-specific cells, and the need for proper vascularization of the tissue models. In addition, limited biomaterials are available for use at this time. Thus, there is a pressing need for novel bioink formulations to improve the fabrication of functional tissue constructs and facilitate their applications in drug testing.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?