Naive Pluripotent Stem Cell Culture in Serum-Free Media
Gabi Tremml, Ming Li and Vi Chu1*1
Merck, Bioscience Division, Temecula, CA, USA,
Read more about
Introduction
Naive pluripotent stem cells are located within the epiblast of mature blastocysts. These primitive “ground-state” cells may be cultured in vitro using specialized media and small molecule inhibitors1. Naive stem cells have several advantages over current “primed-state” pluripotent stem cells including: greater differentiation potential, higher mouse chimera formation, more efficient CRISPR gene editing and wider utility as potential cellular therapies.
Defined serum-free and feeder-free culture of mouse embryonic stem (mES) cells holds many advantages over the classical serum-containing feeder-dependent culture methods, ranging from decreased lot-to-lot variation to ease of culture. The discovery that the inhibition of differentiation inducing signals is critical for mES cell self- renewal even in absence of the cytokine Leukemia Inhibitory Factor (LIF) led to a new definition of the “ground-state” of ES cell self-renewal1. Inhibitors that block MAPK/Erk pathway, in combination with the glycogen synthase kinase 3 (GSK3) inhibitor, protect mES cells from differentiation-inducing signals, allowing for self-renewal in serum-free medium2,3. In this study, we show that the defined serum-free and feeder-free ESGRO®-2i medium can be used for prolonged pluripotent mES culture in naive pluripotent cell states.
Methods
Clonal Mouse ES Cell Assay
mES cells (CMTI-1) were expanded for 1-2 passages prior to low density clonal assay in ESGRO® Complete Plus Clonal Medium (SF001-500P). Cells were split with Accutase™ reagent (A6964), washed twice with ESGRO® basal medium (SF002) and counted. 6-well plates were coated with 0.1% gelatin (ES-006), and wells were seeded with 1000 cells/well in either ESGRO®-2i medium (SF016-200). After 5 days, colonies were counted.
Immunocytochemistry
For Oct-4 staining, we used mouse Oct-4 antibody (MAB4419) at a 1:100 dilution, and secondary donkey anti-mouse IgG-FITC antibody at a 1:500 dilution. For the SSEA-1 staining, we used mouse SSEA-1 antibody (MAB4301) at a 1:50 dilution, and secondary goat anti-mouse IgM-Cy3 antibody at a 1:500 dilution. For the Sox-2 staining, we used rabbit Sox2 antibody (AB5603) at a 1:50 dilution, and secondary goat anti-rabbit IgG-Cy3 antibody at a 1:500 dilution.
Gene Expression Analysis
mES cells and induced pluripotent stem cells (iPSCs) were cultured in a 6-well plate until confluence. Total RNA was extracted using the RNeasy® kit (QIAGEN). Samples were treated with RNase-free DNase prior to reverse transcription. cDNA was synthesized from total RNA with oligo(dT) and random hexamer primers mix provided with the iScript™ cDNA Synthesis Kit (Bio-Rad). PCR reactions were carried out using STEMCCA™ Viral Gene Detection qPCR Multiplex Kits. Expression levels of the viral Oct-4 and endogenous Nanog were normalized to the housekeeping GAPDH gene.
Results
During the development of ESGRO®-2i medium, we first compared 129SvEv mES cell propagation with a low density clonal plating assay in the presence of LIF (ESGRO®-2i) with propagation in the absence of LIF (2i). We found that the presence of LIF in the inhibitor medium led to better colony propagation and greatly enhanced the general health of ES colonies, confirming that pluripotent cells remained LIF-responsive (Figure 1). After culturing 129SvEv mES cells for more than 10 passages in ESGRO®-2i medium, cells were injected into C57Bl6 host blastocysts. The resulting chimeras yielded germ line transmission (Table 1).
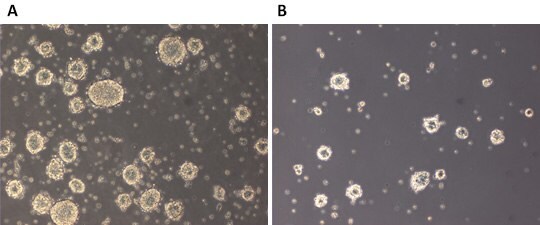
Figure 1. LIF responsiveness of 129SvEv mESCs.Emerging colonies from the low density clonal assay were counted and morphology documented. A) colonies in ESGRO®-2i which contains LIF, GSK3ß inhibitor, and Mek1/2 inhibitor. B) colonies in 2i which contains basal medium with GSK3ß inhibitor, and Mek1/2 inhibitor.
8–15 129SvEv agouti mESCs were cultured for more than 10 passages in ESGRO®-2i medium then injected into C57Bl6 blastocysts. A total of 41 blastocysts were transferred to four donor females. Chimerism was judged according to percentage of agouti coat color. Two 90% chimeras were backcrossed and germ line transmission was confirmed.
We analyzed the pool of injected mES cells for pluripotency markers, both by staining (Figure 2) and by quantitative RT-PCR (Figure 3) and found that relevant markers were expressed. Strikingly, one of the pluripotency markers, mouse Nanog transcript (mNanog), underwent a 6-fold upregulation in mES cells in ESGRO®-2i medium when compared to mES cells cultured in parallel in serum- containing medium on a feeder layer (Figure 3), suggesting that Nanog upregulation is the mechanism by which ESGRO®-2i medium supports pluripotency. Moreover, ES cells could be derived from C57Bl6 blastocysts in ESGRO®-2i medium at an efficiency of more than 10% (data not shown).

Figure 2.Prolonged pluripotent culture in ESGRO®-2i. 129SvEv mESCs were cultured for 10 or more passages. Pluripotency was assessed morphologically using bright field microscopy, A) with anti-Oct4 antibody staining, B) with anti-SSEA1 antibody staining, and C) with anti-Sox2 antibody staining.
We then tested ESGRO®-2i medium for maintenance of pluripotency of induced pluripotent stem (iPS) cells. Distinct states of pluripotency can be specified by culture conditions and are characterized by morphology, signaling pathway dependency, and epigenetic signatures. We hypothesized that ESGRO®-2i medium might provide the culture conditions to aid reversion of pre-iPS cells to the naive state. To test this hypothesis, we generated several mouse iPS cell lines with the STEMCCA™ polycistronic (OKSM) reprogramming lentiviral vector (SCR530). We expanded iPS cells both in ESGRO®-2i medium in feeder-free conditions or in serum-containing medium on feeder layers. To measure the induction of pluripotency, we measured endogenous Nanog expression as well as viral transgene expression represented by lentiviral Oct4 transcripts after 3–5 passages (early passage) and after 10 or more passages (late passage). We found that endogenous Nanog up-regulation was concomitant with viral transgene down-regulation, after extended iPS cell culture in ESGRO®-2i medium. In contrast, neither upregulation of Nanog nor down-regulation of viral Oct4 expression was observed in iPS cells cultured in serum containing conditions on feeder layers (Figure 3). This suggested that ESGRO®-2i medium aids in acquisition of naive pluripotency and may be used to rescue partially reprogrammed iPS cells to a fully reprogrammed, naive pluripotent state.
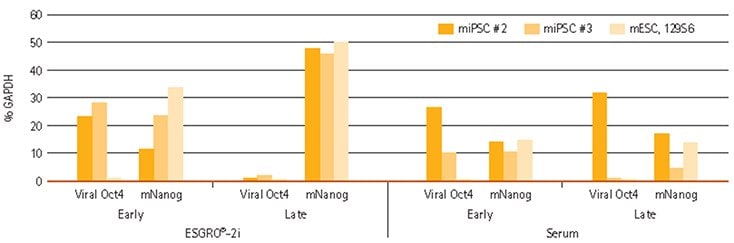
Figure 3.Nanog and Viral Oct4 expression in iPSCs. Viral Oct4 and Nanog transcript levels are shown in 129S6 mESCs at early passages (3–5), and at late passages (>10) in parallel cultures containing ESGRO®-2i medium or serum containing medium in the presence of feeder layers.
Conclusions
Recently, 2i/LIF medium was shown to provide a favorable environment for transitioning pre-iPS cells to naive iPS cells5. This transition was accompanied by increased endogenous Nanog expression while viral transgene expression was down-regulated, highlighting the role of Nanog in facilitating acquisition of pluripotency6. This is consistent with our results, supporting evidence that maintenance of pluripotency in the defined ESGRO®-2i medium is mediated at least in part by Nanog. In summary, we show that the defined serum-free and feeder-free ESGRO®-2i medium can be used for prolonged pluripotent mES cell derivation, maintenance and naive iPSC culture.
Naive Stem Cell Inhibitors
Naive Stem Cell Cytokines
References
To continue reading please sign in or create an account.
Don't Have An Account?