Suzuki-Miyaura Cross-Coupling Reagents
The Suzuki-Miyaura cross-coupling reaction is one of the most important and highly utilized reactions in organic chemistry, with applications in polymer science as well as in the fine chemicals and pharmaceutical industries. However, some classes of boronic acids are exceptionally unstable and susceptible to decomposition which renders them inefficient in coupling reactions or makes long-term storage difficult. Additionally, performing iterative Suzuki Miyaura cross-couplings under mild conditions for the synthesis of small molecules is limited due to the reactivity of boronic acids and therefore a method to allow for iterative couplings under mild conditions has not been previously developed. The mechanism of transmetalation in Suzuki reactions may involve formation of an “ate” complex via interactions between the base and the vacant p orbital on the sp2 hybridized boron atom. Burke and coworkers predicted that a trivalent heteroatomic ligand, such as N-methyliminodiacetic acid (MIDA) (Figure 1) on the boron atom would rehybridize this center to sp3 and thereby attenuate transmetalation under cross-coupling conditions. Release of the reactive, sp2-hybridized boron-species under orthogonal mild conditions would enable this reactivity to be turned back on. In practice, it was discovered that the trivalent MIDA is very effective in this role.1 sp3-Hybridized MIDA boronates are unreactive towards transmetalation (Figure 2: Comparison of sp2 and sp3-hybridized boron species) and the ligand can be cleaved under mild conditions to liberate the corresponding boronic acid. This enables the execution of sequential Suzuki-Miyaura reactions under mild conditions.
The MIDA-protected boronate esters are easily handled, indefinitely bench-top stable under air, compatible with chromatography, and unreactive under standard anhydrous cross-coupling conditions, even at temperatures up to 80 °C. However, deprotection is easily achieved at room temperature under mild aqueous basic conditions using either 1M NaOH, or even NaHCO3.
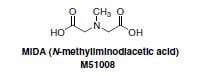
Figure 1.MIDA (M51008)
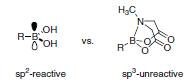
Figure 2.Comparison of sp2 and sp3-hybridized boron species
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?