How to Best Store Electrical Energy
Advantages of Electrochemical Systems
Historically, energy storage to power vehicles and electrical grids has relied on converting chemical energy to mechanical and electrical energy by a heat process using the Carnot cycle. This process often involves burning fossil fuels to generate heat and converting heat to mechanical energy, as in a typical heat engine. Unfortunately, this process is inefficient, as shown by the maximum theoretical efficiency of only 64% and actual efficiencies only at 30-40%. In addition, burning fossil fuels generates environmentally damaging waste products, including CO2, methane, and nitrous oxide. Consequently, scientists have sought more efficient and cleaner energy storage and conversion processes.
Electrochemical systems have tremendous promise for storing energy and converting energy to workable forms. Efficiencies of electrochemical systems typically can be 40-60% and even greater than 85% in newer technologies. In addition, some electrochemical energy storage electrochemical systems, like the polyoxometalate-based redox-flow batteries discussed here, operate with a near 100% atom economy and without generating any chemical waste directly. These aspects give electrochemical systems an advantage compared to heat engines.
Advances in Fuel Cells
One promising type of electrochemical system is the hydrogen-based fuel cell. Assuming hydrogen as a fuel, fuel cells can offer high efficiencies for electricity production of 50–60% compared to a heat engine of 30–40%. Current stack operations yield power densities of 6kW/L and more. If goals of 1 W/cm2 @0.8V (single cell) become feasible, the efficiencies will be pushed to 70–80%.
Hydrogen-based fuel cells are very attractive for energy conversion because they are much more efficient and environmentally cleaner than heat engines. Still, hydrogen-based fuel cells do have limitations for energy storage. First, using hydrogen as an energy storage medium means converting electricity to hydrogen and back to electricity. Because this involves two conversions with losses at each conversion, the efficiency of the storage process drops to only 35–45%. Second, hydrogen fuel cells are expensive. Hydrogen fuel needs specific storage conditions, such as high-pressure tanks and/or cryogenic temperatures. Building hydrogen storage at scale requires significant capital investment considering the components, the electrolyzer, the fuel cell, and other components. Third, hydrogen is highly flammable, which poses safety hazards. The flammability, combined with the difficulties of storing hydrogen, significantly limits the use of hydrogen as a mobile fuel.
Li-Ion Batteries
An alternative electrochemical system, a battery, is much better suited to energy storage. Typical battery storage efficiencies, including the entire cycle, are around 80%, nearly double that of today’s hydrogen fuel cell. Two battery systems, among many more, should be briefly mentioned here. The Li-ion battery has become quite ubiquitous because of its excellent properties. These batteries are used in portable electronics, automotive applications, and stationary purposes. For example, a Li-ion battery of 85kWh in a Tesla Model S car has a volume and weight of 263 L and 400 kg, respectively (excluding protective housing, BMS, and power conditioning), which can yield a range of up to 450 km.
But Li-ion batteries have downsides. One downside of the Li-ion battery is that the raw materials used in the battery are available only in a few countries (similar to oil) with all the polito-economic consequences associated. Another downside is that the Li-Ion battery lifetime (load/unload cycles) is short, and methods to recycle the valuable battery components are still in their infancy. In addition, technical improvements, like the faster charge-discharge rate and increased gravimetric capacity, are needed. One notable downside of lithium-ion batteries is their safety. Under certain operation conditions, but also in standby mode, a thermal run-away can occur, or by other adverse conditions, the batteries can burn or even explode. Many incidents have been described. Recently an ocean freighter bringing electric cars from Europe to North America burned out and sank, with steel walls melting in the high heat of the fire- induced by the Li-ion batteries.
Another type of battery is the redox flow battery (RFB). A redox flow battery, like any battery, converts chemical energy to electrical energy. In a redox flow battery, two solution-phase chemical components are pumped passed current collecting electrodes on separate sides of an ion-selective membrane. The redox chemistry at the electrodes’ surfaces results in a flow of electrons through an external circuit accompanied by ion transfer through the membrane. The chemical potentials of the chemical components determine the cell voltage. At the same time, the energy capacity depends on the electrolyte volume, and the power depends on the surface area of the electrodes.
Unlike a traditional battery, like a Li-ion battery, where storage and conversion both occur in one entity of the battery cell, the electrode, in an RFB, the converter and the container for the “fuel” are separated. This separation of energy and power has advantages regarding the design of such systems, e.g., doubling the capacity of the battery is possible by simply doubling the size of the tank. (Doubling the capacity of a Li-ion battery is much more costly and cumbersome.) In addition, unlike traditional batteries, in an RFB, only a few percent of the total stored energy is connected electrochemically at any one time, which limits the risk of a runaway or uncontrolled energy release. Because flow can easily be stopped during a fault condition, the vulnerability to runaway is significantly reduced. Still, RFBs have their limitations. For example, the most common type of redox flow battery, the vanadium redox flow battery, is limited to a low energy density because the redox process only involves one electron per ion. In addition, it offers a low power density because of the slow rate constant of the process. Finally, vanadium-based RFBs use a highly corrosive and environmentally damaging liquid for operation.
Converting Problems to Advantages in Redoxflow Batteries Using Polyoxometalates (POMs)
Chemical Characteristics
It is highly desirable to have an energy and power capability similar to Li-ion batteries and the flexibility of an RFB concept but with safe and environmentally benign materials and processes. A possibility to achieve this is to use polyoxometalates (POMs) as redox systems in an aqueous solution close to neutral pH. POMs are complex ions with multiple redox centers of the same or different metals. For example, SiW12, which has the formula of [SiW12O40]4-, is an approximately spherical cluster of tungsten atoms bound to oxygen and a central silicon atom (Figure 1). Similarly, PV14 (Figure 2), which has the formula [PV14O42]9-, is a cluster of vanadium atoms bound to oxygen and a central phosphorus atom. In an RFB, POMs offer several advantages over dissolved metals like vanadium: (1) POMs can deliver multiple electrons, (2) POMs offer relatively high solubilities (often >0.5 M), which enables higher energy content; (3) POMs are typically very stable, capable of withstanding more than 20,000 charge-discharge cycles without significant degradation; (4) Because POMs have delocalized electron density, electron transfer is very fast (e.g., for PV14, it is 104 times faster than for the V ion itself); and (5) POMs are made of environmentally benign and inexpensive materials.
An RFB battery based on POMs offers high energy density and high-power density at the same time. Coupled with the advantages of inexpensive and environmentally benign materials, POM-based RFBs have applications in three fields: mobility, power grid, and decentralized storage.
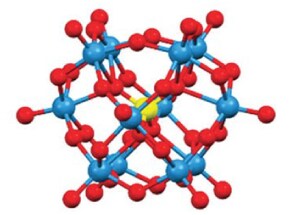
Figure 1.Model of SiW12.
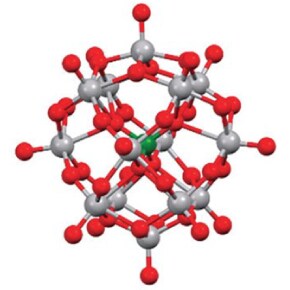
Figure 2. Model of PV14.
Mobility
Mobility is currently a hot topic regarding electrification. For automobile battery solutions, Li-ion batteries are presently favored. There are various challenges with the technology, as already noted, but limited resources for materials, limited power capabilities, and missing infrastructure are the most severe ones. Some of these challenges are so severe that using lithium-ion batteries in electric trucks is questionable. With the one liquid POM-RFB, we can achieve energy densities at par with Li-ion, but power densities are up to 50% higher than with Li-ion.
The following example illustrates the situation: If you take a Li-ion battery in a Tesla Model S vehicle of 85 kWh, the whole system has a volume and mass of 263 l and 400 kg, respectively. A one-liquid POM system of the same capacity has a volume of 200 l and a mass of 400 kg. The resulting specific values are 0.21 kWh/kg for both and 0.43 kWh/L (POM) and 0.32 kWh/L (Tesla). Considering the power capabilities, the comparison is 0.38 kW/kg and 0.75 kW/L (POM) and 0.21 kW/kg and 0.31 kW/L (Tesla), respectively (see Table 1). Considering even some variations in numbers, performance data are at par, with a somewhat better power density for the POM system.
However, the most striking advantage for RFBs in mobile energy storage applications is that the charging process consists of exchanging the spent solution with a fresh one, comparable to the current process of refueling your car with gasoline. This process is faster than the slow charging times for current LIB batteries. It could leverage the existing fuel storage and distribution infrastructure, in contrast to LIB battery technology, which requires new infrastructure and capital investment to create the infrastructure. The RFB concept also enables electric-powered trucks to avoid extra weight problems with scaled-up lithium-ion batteries and their long charging times.
Power Grid
RFBs can also find use in storing energy to stabilize the power grid. In most countries, power plant capacity is laid out to cover all peak loads that can occur in the electric grid. The most common ones are the typical morning and evening peaks. Providing extra electricity during peak times requires large amounts of electricity and fast response. The state-of-the-art vanadium-based RFBs are too slow to respond to the changes in the grid, but POM-based RFBs have power capabilities about 104 times larger than vanadium-based RFBs. This allows for large electricity transfers in the sub-ms regime, a necessity for a fast frequency stabilization of the electric grid. A rough estimate shows that, e.g., in Germany, 20 to 30% of the power plants could be shut down due to this enormous load leveling effect. Beneficial for the environment, this could be a shutdown of most coal-fired power plants. In addition, such storage power stations provide grid stability by integrating renewable energy resources. Due to the extremely low self-discharge rates, it can provide a constant supply to the grid from unstable sources like solar or wind power over days or weeks.
Decentralized Storage
RFBs may provide a solution to decentralized energy storage as well, energy off grids. In an area of increased importance, the local building-related energy storage based on PV and/or small wind turbines, this concept can be beneficial. An RFB is comparable in performance to Li-ion batteries and is much safer because the risk of uncontrolled energy release is significantly reduced. In addition, RFBs may offer longer lifetimes and lower costs.
Conclusions
We advanced to an essential level in the current search for the holy grail of electricity storage. While validation is still needed, the POM-based battery systems tick the boxes of many aspects important for electricity storage, like capacity, power, ease of operation and transport, durability, sustainability, and, eventually, low cost of storing electricity. There may be better systems in the future. Still, this technology allows us to broadly introduce and use energy storage for many applications making fossil fuels more and more obsolete.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?