Gabapentin Assay: Ph. Eur. Monograph
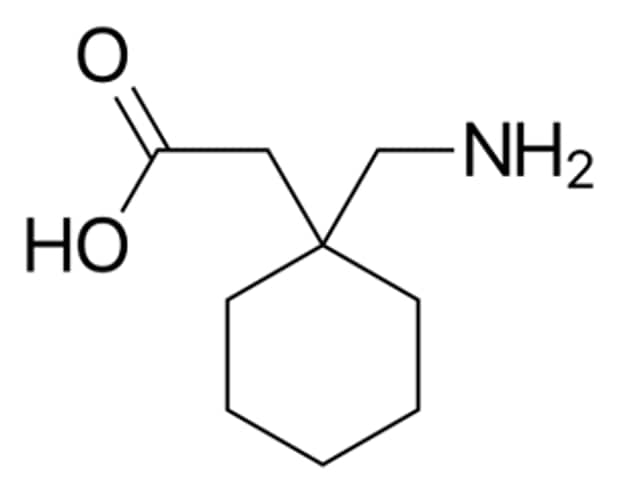
Figure 1.Chemical structure of Gabapentin.
Gabapentin is an anticonvulsant medication primarily used to treat partial seizures and neuropathic pain. It is a first-line medication for the treatment of neuropathic pain caused by diabetic neuropathy, post-therapeutic neuralgia, and central neuropathic pain.1 It is moderately effective - about 30-40% of those given gabapentin for diabetic neuropathy or post-therapeutic neuralgia have a meaningful benefit. Gabapentin acts by decreasing the activity of a subset of calcium channels.2
We demonstrate the assay and related substances determination of gabapentin following the Ph. Eur monograph using the Purospher® STAR RP-18 end-capped column, 250 x 4.6 mm, 5 μm.3
Read more about
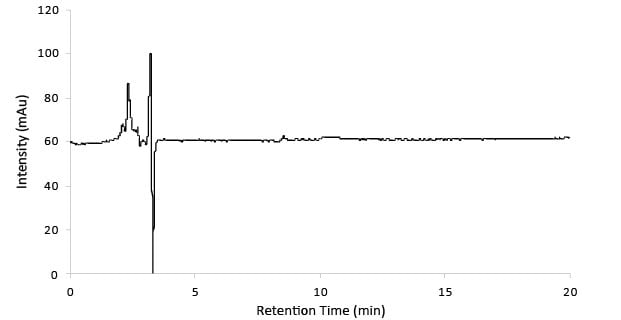
Figure 1.Blank chromatogram.
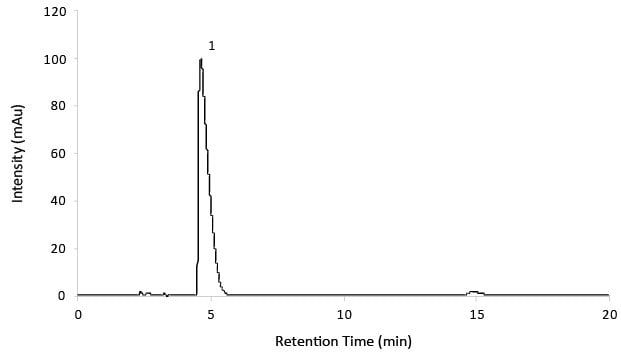
Figure 2.Reference solution C for System suitability.
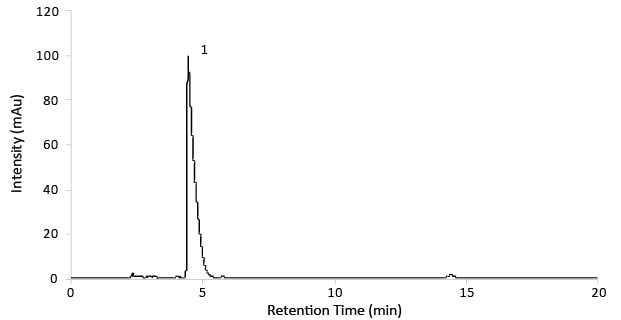
Figure 3.Chromatogram of Gabapentin test solution (14mg/mL).
Gabapentin Assay - Calibration
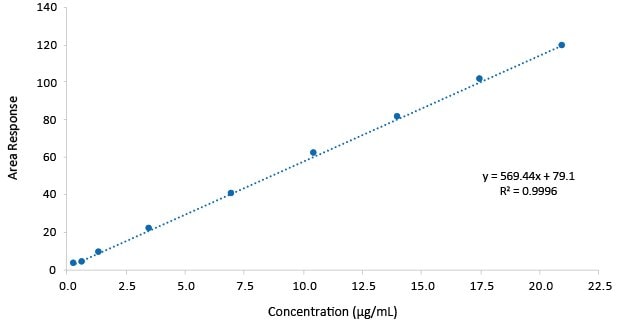
Figure 4.Calibration function Gabapentin.
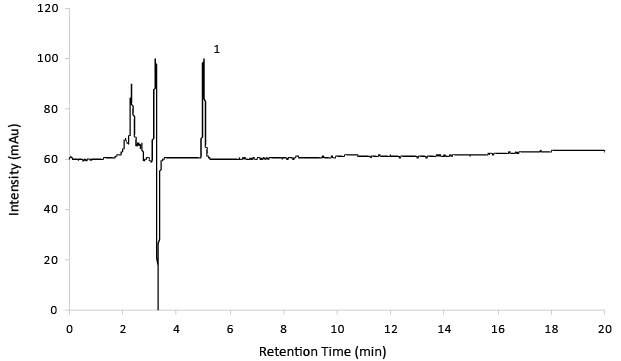
Figure 5.Reference solution A (14 mg/mL Gabapentin).
Run time for impurity testing to be 4x retention time of Gabapentin = 20.06 min.
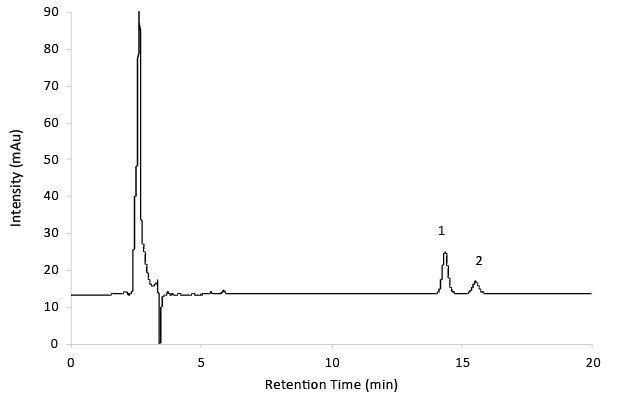
Figure 6.Reference solution B - Gabapentin impurity A and B.
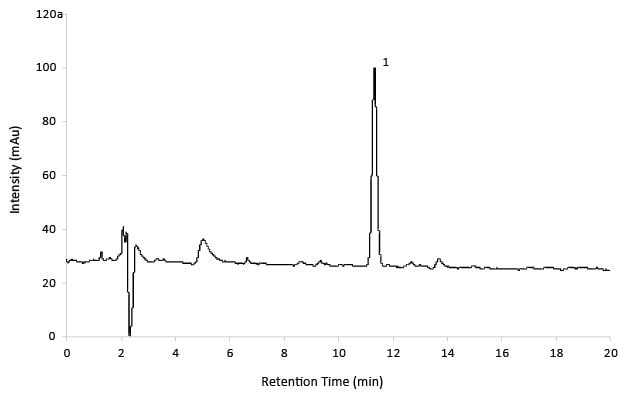
Figure 7.Reference Solution Gabapentin Related Substance D.
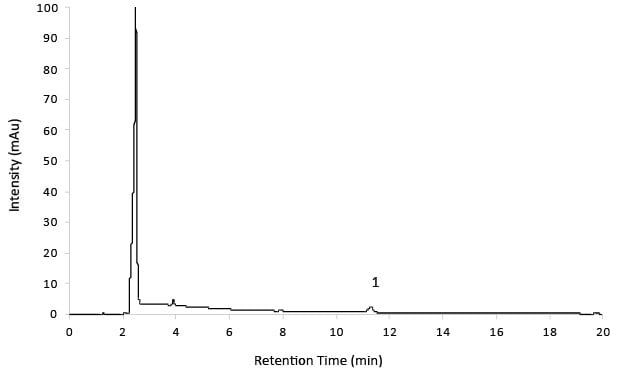
Figure 8.Test Solution 14 mg/mL substance showing 7.0 µg/mL Impurity D.
Chromatographic Data (Test Solution: 14 mg/mL Substance Showing 7.0 µg/ mL Impurity D)
System Suitability
Recovery of Gabapentin Related Substances A, B, D and E Spiked in Test solution (14 mg/mL), using Purospher® STAR RP-18e
Conclusion
The assay and related substances determination of gabapentin in pharmaceutical preparations with the Ph. Eur. monograph3 is demonstrated with the Purospher® Star RP-18 endcapped column, 250 X 4.6 mm, 5 μm.
The assay method shown, provided for gabapentin a Limit of Detection (LOD) of 0.55 µg/mL, and a Limit of Quantification (LOQ) of 1.68 µg/mL with linearity to a concentration of up to 21.0 µg/mL. The RSD on the repeatability of the gabapentin assay was <2%.
For the related substances A, B, D, and E of gabapentin, the Limit of Detection (LOD) ranged from 0.52 to 2.0 µg/mL and the Limit of Quantification (LOQ) was 1.58 to 6.06 µg/mL. Percentage recoveries ranged from 96.5 to 109.9%.
See more information on quality control testing at SigmaAldrich.com/PharmaQC
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?