Building Regional Cell Culture Media Supply Chains
Regionalization of Manufacturing to Ensure Consistent and Continuous Supply of Essential Cell Culture Media
We're investing in regional manufacturing with built-in redundancy, so you will never have to worry about having a reliable supply of consistent, high-quality cell culture media.
Volatile global supply chains put biopharma manufacturing at risk. That’s why we’ve reimagined our supply chain by building a hybrid global-local manufacturing network for cell culture media.
Combining global oversight with a regionalized supply chain delivers reliable, consistent cell culture media and minimizes the risk of supply disruptions - supporting your business continuity.
Stay ahead by subscribing to our newsletter, which includes updates on our supply network, new products and services, and best practices.
Section overview
- A Global Network of Redundant Regional Manufacturing Sites
- A Globally Harmonized Quality System, for Certified, Consistent Quality - Worldwide
- Quality Assurance Processes Beyond the Standards
- Proven Manufacturing Process Comparability
- Consistent Portfolio Around the Globe
- Lower Emissions, Same High Standards
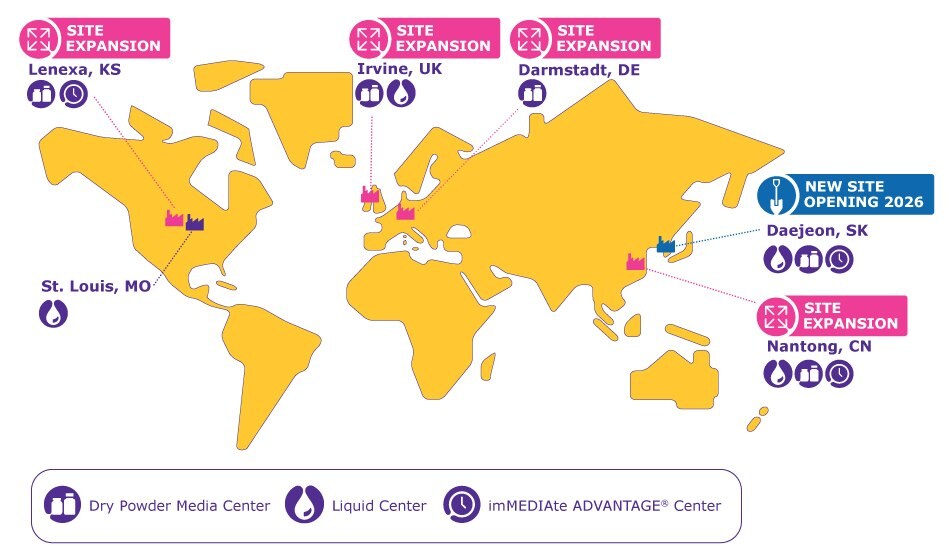
Map 1.Cell culture media production footprint for drug development and manufacturing.
A Global Network of Redundant Regional Manufacturing Sites
Beyond recently completed expansions of our established production sites - adding a third dry powder manufacturing line in Lenexa, Kansas, USA, a new dry powder media line in Nantong, China, and a new production line for custom dry powder media in Darmstadt, Germany - we're expanding our manufacturing footprint around the world. This includes a new site in Daejeon, South Korea, to address increasing demand from the growing biomanufacturing sector in Asia, and in total:
- 11 dry powder GMP manufacturing lines supporting batches from 25 to 8,000 kg
- Liquid cell culture media manufacturing lines supporting batches from 50 to 10,000 L
- Gamma-sterilized antifoam lines in St. Louis, Missouri, USA and Daejeon, South Korea
- Animal-component-free (ACF) manufacturing lines
- All sites* certified for EXCiPACT® GMP standards for Pharmaceutical Auxiliary Materials (PAM)
This is part of our goal to build a regional supply strategy that brings manufacturing closer to your operations, meaning:
- Increased production capacity and more production lines for dry and liquid media needs, and cell culture media ingredients such as EX-CELL® Antifoam foam control agent
- Dedicated regionalized capabilities
- Streamlined logistics with shorter transportation distances
- Minimized risk for many types of potential supply disruptions
Each site uses the same core manufacturing processes and production technologies, and applies a single, globally approved and managed raw material supplier list and qualification approach. As a result, you get the same trusted cell culture media – with the same product specifications - from a site closer to you, with local support when you need it.
A Globally Harmonized Quality System, for Certified, Consistent Quality - Worldwide
Switching suppliers for essential media and materials due to a supply disruption threatens business continuity and can lead to costly delays while you conduct investigation studies. Our global network of redundant manufacturing sites offers the business continuity you need for today’s challenging supply situations. Most importantly: Regardless of which site produces your cell culture media, you can expect comparable product performance, specifications, and lot-to-lot consistency - supporting a reliable quality of your final product***. This is achieved through:
- Qualified production processes backed by one globally harmonized quality system: Every factory uses the same core production processes, technology, and raw materials from a single, globally approved supplier list, proven by comparability studies - ensuring consistent cell culture media quality across our network of regional manufacturing sites.
- Certification according to EXCiPACT® GMP standards for Pharmaceutical Auxiliary Materials (PAM): A new industry standard for raw materials that we co-developed to ensure harmonized and certified quality across all production sites producing cell culture media.
- Emprove® Dossiers: Available for a wide range of products, they provide comprehensive documentation to facilitate your qualification, risk assessment, process optimization, and regulatory compliance.
Quality Assurance Processes Beyond the Standards
We go beyond industry standards with a unique framework for consistency and quality. Our integrated approach significantly reduces the risk of variability, ensures high-quality outputs, and establishes a robust framework for continuous improvement. This includes:
1. Unified Foundation: Our commitment to quality and consistency is anchored in utilizing the same core processes and technology, one quality standard, and a single approved supplier and raw material list across our manufacturing network.
2. Rigorous Management and Improvement Cycle: We adhere to a comprehensive cycle of managing, mitigating, and monitoring:
- Manage: We conduct scientific studies on raw materials and internal manufacturing processes, and constantly execute a Holistic & Proactive Raw Materials and Suppliers Risk Assurance Program. This enables us to identify risks early and determine the need for mitigation activities or adjustments to our supply chains.
- Mitigate: We consult with our customers to recommend enhancements to their formulations, aiming to improve characteristics that reduce supply chain vulnerabilities, address trace metal risks, and enhance chemical-physical parameters without altering their formulations. We also offer innovative in-house raw materials that eliminate specific inherent challenges associated with certain raw materials.
- Monitor: Our routine manufacturing and supply chain activities are continuously monitored by global and cross-functional review boards, ensuring the quality and effectiveness of our raw material supply chains and manufacturing processes. This monitoring process enables us to trigger corrective actions or activities as needed, fostering a culture of continuous improvement and ensuring we adapt to any emerging challenges. Additionally, we employ a parallel approach by assessing high-risk raw materials. This involves defining enhanced fit-for-purpose specifications and implementing dedicated trending analyses for trace metals and other critical parameters.
Proven Manufacturing Process Comparability
As we add new cell culture media facilities around the globe, we conduct comparability studies to ensure the manufacturing processes and equipment used at the given sites produce cell culture media that are comparable across all cell culture media manufacturing facilities.
For those studies we use two complex chemically defined, in-house media formulations that present formulation and milling challenges. The study conditions across the sites are identical, with identical sampling methods and identical validated testing, performed using same qualified laboratories and producing minimum, maximum, and average range batch sizes per production line. All samples are quantitatively analyzed for component recovery against theoretical formulation and homogeneity.
In addition to the comparability evaluation, a formal risk assessment comparing the manufacturing equipment and process parameters is performed.
As we move forward in the design, installation, and qualification of our new site in Daejeon, South Korea, and other production site expansions, we will carry out the same studies to prove comparability.
Consistent Portfolio Around the Globe
This global standardization allows us to offer a comprehensive portfolio of consistent cell culture media and ingredients, including:
- Dry Powder and liquid cell culture media: Ready-to-use and available in a variety of standard or custom volumes, our catalogue media portfolio supports upstream bioprocessing workflows from development through commercial production.
- Custom formulations: From early-stage development to commercial scale, our custom media are precisely engineered to match your process requirements and cellular performance targets, supported by deep technical expertise, traceable raw materials, and flexible manufacturing capabilities.
- Cell culture media ingredients such as EX-Cell® Antifoam and HTST treated glucose – our sterile liquid solutions**** provide the efficiency and flexibility you need in your bioprocess.
- Pre-GMP small-scale custom media, feeds, supplements, and buffers: Our imMEDIAte ADVANTAGE® services for pre-GMP small-scale custom media, feeds, supplements, and buffers provide the quick turnaround you need to accelerate your development work—to get your molecule to the clinic faster.
Lower Emissions, Same High Standards
Sustainability isn’t an extra—it’s embedded in how we build and operate:
- Daejeon’s site will use a variety of resource-efficient equipment, including solar panels for electricity, a heat-pump system for heating, energy-efficient equipment and automation, and water collection systems to reduce water use.
- Shipping to customers in the Asia-Pacific region from Daejeon results in shorter routes, and enables a shift from air to road, reducing CO2e emissions from transportation as much as 98%.
- Offering Greener Alternative Products, like the Cellvento® ModiFeed Prime COMP can further help customers reduce their environmental footprint.
We take a holistic, life-cycle approach to sustainability, both by implementing new manufacturing standards and supporting our customers in achieving their own sustainability goals.
*The new Daejeon manufacturing site is planned to receive certification in accordance with EXCiPACT® GMP standards for Pharmaceutical Auxiliary Materials (PAM) by 2027.
** EXCIPACT GMP
*** Comparability studies for the new Daejeon manufacturing site are currently underway.
**** Apart from our low bioburden Cleaning -in-Place Solutions and gamma-sterilized Antifoam, our liquids solutions are sterile filtered.
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?