Daejeon, Engineered to Global Standards, Manufacturing Next Door for Business Continuity
We are expanding our global network of regional manufacturing sites, to meet the growing demand for bioprocessing solutions in Asia-Pacific.
Our new 44,500 m² Bioprocessing Production Center in Daejeon, South Korea, set to open in Q4 2026, marks a significant expansion of our manufacturing capacities and capabilities.
Complementing existing manufacturing sites, it will offer the same trusted biotech products—with the same product specification, performance, and same quality standards—from a site closer to you, with local support when you need it. By increasing production capacity with dedicated regional capabilities and streamlining logistics through shorter transportation distances, the Daejeon site will contribute to a more efficient supply chain, ensuring business continuity.
Discover our factory's capabilities, sustainability efforts, and commitment to quality. Click 'Download brochure' to learn more.
Explore Products and Services Available From Daejeon, South Korea
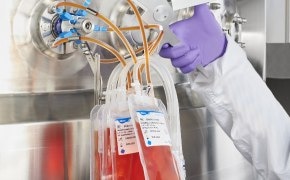
Reliable Sampling in Bioprocesses
Experience reliable and representative sampling, backed by comprehensive documentation supporting qualification, risk assessment and regulatory approval. Request your free sample!

Custom Cell Culture Media and Buffer Services
Leverage our long-term expertise to optimize your cell culture solutions and achieve the performance and quality criteria you need to scale with confidence. Test your prototype!

Liquid Buffers and Cleaning-in-Place Solutions
Optimize your bioprocessing with our high-quality products. Backed by comprehensive documentation, they come with essential information for risk assessment and regulatory approval. Request your free sample!

Dry and Liquid Cell Culture Media
Discover our catalog media and feeds, or benefit from custom media, soon to be produced in Daejeon. Experience consistent quality and performance, enhancing your cell culture processes. Explore Cell Culture Media
Part of a Global Network of Regional Manufacturing Sites
Beyond recently completed expansions of our established production sites, we're developing our manufacturing footprint around the world, including the new Daejeon site. This global-local supply strategy addresses the vulnerability of biopharmaceutical supply chains, providing regionalized manufacturing redundancy, ensuring business continuity.
Adhering to One Global Quality System
Like all our factories in the world, our Daejeon site will use the same core production processes, technology, and raw materials from a single, globally approved supplier list, and redundancy will be proven by comparability studies—ensuring consistent product quality. The site is planned to receive certification in accordance with EXCiPACT® GMP standards for Pharmaceutical Auxiliary Materials (PAM) by 2027.
Comprehensive documentation to facilitate your qualification, risk assessment, and process optimization efforts is available as part of our Emprove® Program for various products made in Daejeon, including buffers, cleaning-in-place solutions, cell culture media, and sampling systems.
Designed for Smart Manufacturing
The Daejeon site will continuously implement smart manufacturing technologies over time, leveraging digital technologies like Internet of Things (IoT), AI, and big data to optimize production processes. In addition to digital innovation, Daejeon will apply advanced technologies such as automated guided vehicles, automated warehouse and industrial robots for operational efficiency.
Key features will include interconnected machines and sensors for real-time data exchange to business operations systems, automation for increased efficiency, and data analytics for real-time data driven decision-making. This will enable flexibility in adapting to changing demands and will increase efficiency.
Embedding Sustainability
We will use a variety of resource-efficient equipment including solar panels for electricity, a heat-pump system for heating, energy-efficient equipment and automation, and water collection systems to reduce water use. Shipping to APAC customers from Daejeon will result in shorter routes, which can reduce CO2e emissions from transportation by up to 98%.
Production
Capacity
Regionalized
Capabilities
Logistics
Performance,
Quality Standards
Manufacturing
Related Webinars
Watch our webinars for more insights on our supply strategy and products and services available from Daejeon.
To continue reading please sign in or create an account.
Don't Have An Account?

