Grignard Reagents
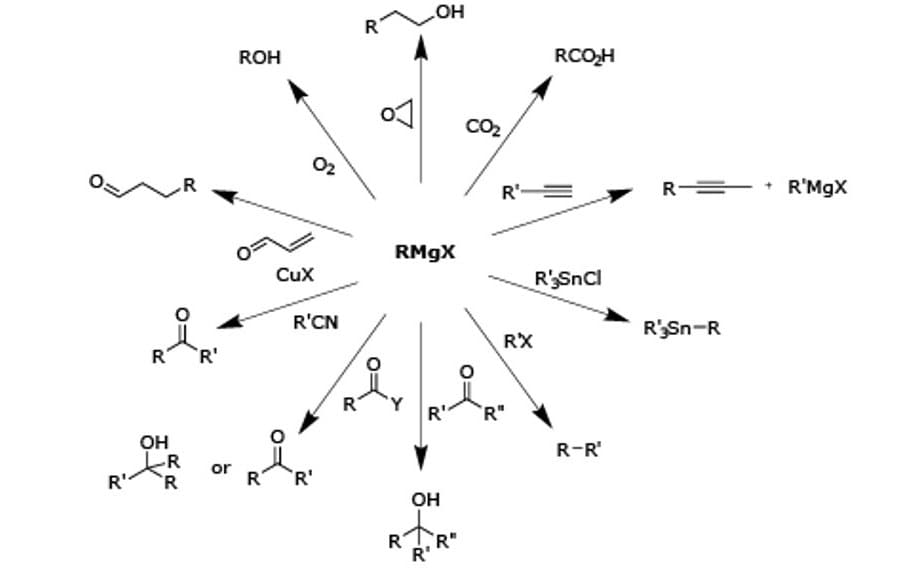
Grignard reagents are highly reactive organomagnesium halides formed by the reaction of magnesium metal with alkyl or alkenyl halides. They are very strong bases and react with acidic hydrogens such as alcohols, water and carboxylic acids. Our comprehensive portfolio of Grignard reagents, used in the Grignard reaction to form new carbon-carbon bonds, will make your breakthroughs feel closer than ever.
Products
What is a Grignard reagent?
A Grignard reagent is an organomagnesium halide having a formula of RMgX, where X is a halogen (-Cl, -Br, or -I), and R is an alkyl or aryl (based on a benzene ring) group. To initiate a Grignard Reaction, a Grignard reagent is added to a ketone or aldehyde, to form a tertiary or secondary alcohol. The reaction with formaldehyde leads to a primary alcohol.
Grignard reagents can be used for determining the number of halogen atoms present in a halogen compound. Grignard degradation is used for the chemical analysis of certain triacylglycerols as well as many cross-coupling reactions for the formation of several carbon-carbon and carbon-heteroatom bonds. Industrially, the Grignard reaction is the key step in the production of Tamoxifen, which is used in the treatment of breast cancer.
Making a Grignard reagent
Grignard reagents are produced from the heated combination of halogenoalkane and magnesium in the presence of diethyl ether (ethoxyethane). The reaction should be kept dry to avoid the resulting Grignard reagent from reacting with water.
Related Resources
- Selective 1,2-Additions with LaCl3·2LiCl
Grignard reagent's 1,2-addition to ketones is powerful but selective issues often arise from competitive alpha-deprotonation.
- Selective Metalations
Salt additives increase both the rate and the efficiency of the Mg-halogen exchange reaction. The most effective reagents are generated with R-MgCl (R = i-Pr, s-Butyl) and 1.0 equiv of LiCl.
- Aldrichimica Acta VOL. 41 NO.3: Green, Mild, And Versatile Synthetic Methods
Aldrich® Is Pleased to Offer Cutting-Edge Tools for Organic Synthesis
- Aldrichimica Acta VOL. 48 NO.2: Late Stage Functionalization
Late Stage Functionalization (LSF) allows synthetic and medicinal chemists to structurally modify one Lead Compound into a small library of analogs for further screening and eventual optimization.
- Aldrichimica Acta VOL. 48 NO.3: Spirocyclic Modules
Spirocyclic modules containing four-membered rings are currently of growing interest to discovery chemists.
To continue reading please sign in or create an account.
Don't Have An Account?