Subculture of Adherent Cell Lines
Adherent cell lines will grow in vitro until they have covered the surface area available or the medium is depleted of nutrients. At this point the cell lines should be subcultured or passaged in order to prevent the culture dying. To subculture the cells they need to be brought into suspension. The degree of adhesion varies from cell line to cell line but in the majority of cases proteases, e.g. trypsin, are used to release the cells from the flask. However, this may not be appropriate for some lines where exposure to proteases is harmful or where the enzymes used remove membrane markers/receptors of interest. In these cases cells should be brought into suspension into a small volume of medium mechanically with the aid of cell scrapers.
Read more about
Subculture Protocol Overview
Assess Cultures
Remove Spent Medium
Incubate for 2-10 Minutes
Examine Cells
Re-suspend Cells in Fresh Medium
Transfer Cells into Fresh, Warmed, New Media
Incubate as Required
Repeat as Necessary
Materials
- Cell Culture Media: pre-warmed to the appropriate temperature (refer to the ECACC cell line data sheet for the correct medium and temperature.)
- 70% (v/v) alcohol in sterile water (793213)
- PBS without Ca2+/Mg2+ (D8537)
- 0.05% trypsin/EDTA in HBSS, without Ca2+/Mg2+ (T3924)
- Soybean Trypsin Inhibitor (T6522) or FBS
- Trypan Blue Solution (T8154)
Equipment
- Personal protective equipment (sterile gloves, laboratory coat, safety visor)
- Waterbath set to appropriate temperature
- Microbiological safety cabinet at appropriate containment level
- Incubator
- Pre-labelled flasks
- Inverted phase contrast microscope
- Centrifuge
- Hemocytometer or automated cell counter like a Scepter™ Cell Counter
- Marker Pen
- Pipettes
- Ampoule rack
- Tissue
Subculture Procedure

- View cultures using an inverted microscope to assess the degree of confluency and confirm the absence of bacterial and fungal contaminants.
- Remove spent medium.
- Wash the cell monolayer with PBS without Ca2+/Mg2+. Repeat this wash step if the cells are known to adhere strongly.
- Pipette trypsin/EDTA onto the washed cell monolayer using approximately 1 ml per 25 cm2 of surface area. Rotate flask to cover the monolayer with trypsin. Decant the excess trypsin.
- Return flask to the incubator and leave for 2-10 minutes.
- Examine the cells using an inverted microscope to ensure that all the cells are detached and floating. The side of the flasks may be gently tapped to release any remaining attached cells.
- Resuspend the cells in a small volume of fresh serum-containing medium to inactivate the trypsin. Remove 100-200 μL and perform a cell count. In the case of cells cultured in serum-free media, use a trypsin inhibitor such as soybean trypsin inhibitor to inactivate the trypsin.
- Transfer the required number of cells to a new labelled flask containing pre-warmed medium (refer to the appropriate ECACC Cell Line Data Sheet for the required seeding density).
- Incubate as appropriate for the cell line.
- Repeat this process as demanded by the growth characteristics of the cell line.
Key Points
- Some cultures, whilst growing as attached lines, adhere only lightly to the flask, thus it is important to ensure that the culture medium is retained, and the flasks are handled with care to prevent the cells detaching prematurely.
- Although most cells will detach in the presence of trypsin alone EDTA enhances the activity of the enzyme by removing inhibitory cations.
- Trypsin is inactivated in the presence of serum. Therefore, it is essential to remove all traces of serum from the culture medium by washing the monolayer of cells with PBS without Ca2+/Mg2+.
- Cells should only be exposed to trypsin/EDTA long enough to detach cells. Prolonged exposure could damage cell surface receptors.
- Trypsin should be neutralized with serum prior to seeding cells into new flasks otherwise cells will not attach.
- Trypsin may also be neutralized by the addition of soybean trypsin inhibitor, where an equal volume of inhibitor at a concentration of 1mg/ml is added to the trypsinized cells. The cells are then centrifuged, resuspended in fresh culture medium and counted as above. This is especially necessary for serum-free cell cultures.
- If a CO2 incubator is not available gas the flasks for 1-2 minutes with 5% CO2 in 95% air filtered through a 0.2 μm filter.
- If the cells harvested are at too low a cell density to re-seed at the appropriate cell density into fresh flasks it may be necessary to centrifuge the cells e.g. 5 mins at 150 x g, and resuspend in a smaller volume of medium.
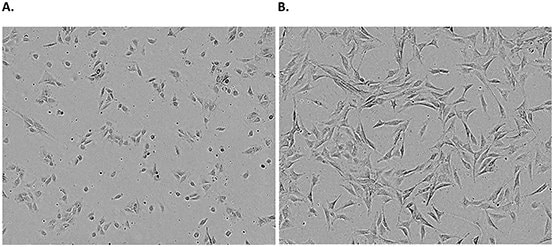
Figure 1.Examples of an adherent cell line. NIH 3T3 cells 24 hours (A) and 72 hours (B) post freeze/thaw show typical adherent fibroblastic morphology. Confluence should carefully be monitored and passaged when cells reach ~80% confluency.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?