SeqPlex Enhanced DNA Amplification Kit (SEQXE) Protocol
Product Description
The SeqPlex DNA Amplification Kit (SEQXE) for whole genome amplification (WGA) is designed to facilitate next-generation sequencing (NGS) from extremely small quantities or from degraded/highly fragmented DNA. The yields from chromatin immunoprecipitation (ChIP) or formalin-fixed paraffin-embedded tissue samples (FFPE) are often less than required for successful NGS library preparation. The SeqPlex kit allows the user to pre-amplify these and other small quantity/highly fragmented DNA samples for input into a NGS workflow.
The SeqPlex process is comprised of three steps: pre-amplification, amplification and primer removal. See SeqPlex Process Workflow chart below.
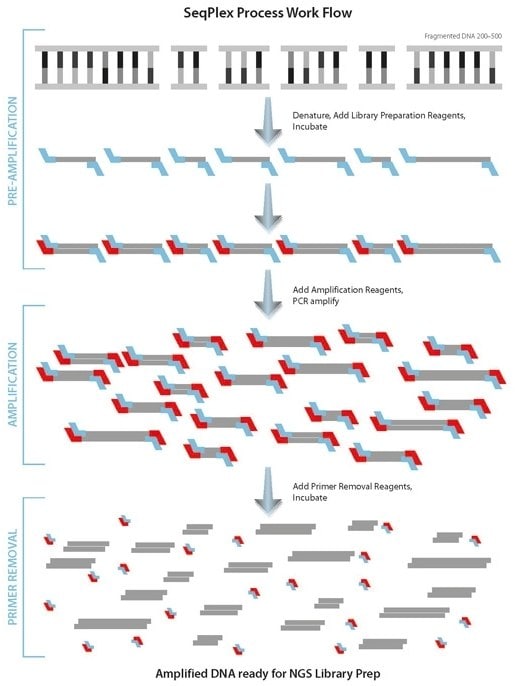
In the first step, Pre-Amplification, the DNA is replicated using primers composed of a semi-degenerate 3’-end and a universal 5’-end. As polymerization proceeds, displaced single strands serve as new templates for additional primer annealing and extension. The Pre-Amplification product is composed of random, overlapping amplicons which are flanked by a universal end sequence.
In the second step, Amplification, the product of Pre-Amplification is amplified by single primer PCR via the universal end sequence. The product of SeqPlex amplification ranges from 200 to 500 base pairs. Amplicons from ChIP and/or degraded DNA, such as FFPE, are typically shorter and dependent upon length of the starting DNA.
The third step, Primer Removal, removes the primer and semi-degenerate sequences from the amplicon pool making SeqPlex DNA ready to enter a NGS workflow.
Reagents Provided
Storage/Stability
All components should be stored at –20 °C. When thawed for use, components should be kept on ice. Dissolve any precipitate in these solutions by briefly heating at 37 °C, with thorough mixing. Stability of the Library Preparation Enzyme and Primer Removal Enzyme will be affected if stored above –20 °C or allowed to remain for long periods at temperatures over 4 °C
Procedure
The following procedure has been used successfully to amplify and sequence 100 pg of ChIP-isolated DNA and 100 pg of fragmented genomic DNA. Damaged DNA, such as that isolated from FFPE tissues, may require 5- to 10-fold more input DNA, depending on the degree of damage. Reactions can be scaled up or down to accommodate preparation of needed quantities of amplified DNA.
Note: Final yield after amplification and primer removal varies significantly depending upon the quality of starting DNA. In most cases, 1 to 2 μg can be expected. Perform multiple reactions for larger quantities.
Note: This procedure was developed using the specific reagents provided with, or recommended for use with, this kit. Substitutions may result in suboptimal results.
Pre-amplification
- For the best SeqPlex amplification coverage and NGS results, the optimum starting DNA size is 200-500bp. DNA from many ChIP and FFPE samples are already within this size range. Such samples do not require additional fragmentation prior to step 4 of the SeqPlex protocol.
If starting DNA is not within the size range of 200-500 bp, fragmentation is strongly recommended. DNA may be fragmented using sonication, a Covaris instrument, or enzymatic fragmentation.
- If possible, determine the concentration of DNA by UV absorption (260 nm), and use up to 1 ng per reaction.
- Thaw the Library Preparation Buffer (LP100), 5x Amplification Buffer (A5112) and water (W4502). Mix thoroughly.
- Combine as follows:
2 µL of Library Preparation Buffer (LP100)
X µL DNA (up to 1 ng)
Y µL water (W4502)
--------------------------------------------------
14 µL Total reaction volume
Caution—Experienced GenomePlex® WGA users: Several components found in the SeqPlex Enhanced DNA Amplification kit (SEQXE), SeqPlex DNA Amplification kit (SEQX), GenomePlex WGA kits, Transplex® WTA1 kit and Complete Whole Transcriptome Amplification Kit (WTA2) are similarly named. Though generally analogous in function, they are not interchangeable.
- Mix thoroughly, centrifuge briefly, and incubate in a thermal cycler programmed for:
95 °C for 2 minutes
4 °C Hold
- After the sample has cooled to 4 °C, add 1 µL of Library Preparation Enzyme (E0531). Cap tube and mix thoroughly. Centrifuge briefly and immediately proceed to next step.
- Place reaction in a thermal cycler and incubate as
follows:
16 °C for 20 minutes
24 °C for 20 minutes
37 °C for 20 minutes
75 °C for 5 minutes
4 °C Hold
- Remove sample from thermal cycler and centrifuge briefly. Amplification may be completed immediately or store Pre-Amplification product at –20 °C for up to three days.
Caution—Experienced GenomePlex WGA users:
· SeqPlex uses a 5x Amplification Mix
· SYBR Green (S9430) is recommended to monitor the amplification
· Annealing/Extension temperature is 70 oC
· A 30-minute 70 oC post-amplification hold is required
- Add the following reagents to the 15 mL of Pre-Amplification product from step 8. (For multiple reactions, a master mix may be prepared. Add 60 mL of the master mix to each reaction):
15 µL 5X Amplification Mix (A5112)
1.5 µL DNA Polymerase for SeqPlex (SP300)
42.5 µL Water (W4502)
1 µL SYBR Green I (S9430) diluted 1/1000*
- µL Instrument Specific Reference Dye (optional)
--------------------------------------------------
75 µL Total reaction volume
*For the best representation, real-time PCR with addition of SYBRGreen I to the amplification reaction is strongly recommended to enable monitoring of the reaction progress. SYBRGreen I (S9430) must be diluted 1,000-fold (1/1000) and 1µl used per 75 µl SeqPlex amplification reaction to avoid inhibiting the amplification reaction. SYBRGreen formulations other than S9430 have not been tested and are not recommended.
Optimal results are achieved by proceeding at least 2–3 cycles after the start of the amplification “plateau”, as indicated on Figure 1. The optimal number of amplification cycles varies with starting DNA template quantity and quality.
If amplification is performed without adding SYBRGreen I, 18 to 24 cycles usually gives good results with 0.1-1.0 ng of high quality DNA. Low quality DNA may require higher input quantities and/or more cycles. If input amounts are near
10 pg, as many as 29 cycles may be required to reach amplification plateau.
Note: If more than 29 cycles are required to achieve plateau, subsequent NGS results may be unsatisfactory. Consult the Troubleshooting Guide.
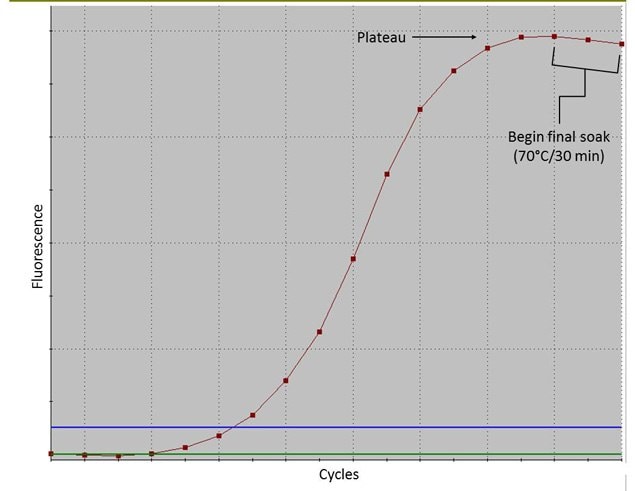
10. Mix thoroughly and cycle in a real-time thermal cycler as follows:
Initial Denaturation: 94 °C for 2 minutes
Cycle until 2-3 cycles into plateau:
94 °C Denature for 15 seconds
70 °C Anneal/Extend for 5 minutes
(read fluorescence)
After cycling:
70 °C for 30 minutes
4 °C Hold
Note: The extended incubation at 70°C after cycling is absolutely essential for efficient primer removal.
Reactions may be purified immediately or stored at –20 °C until purification.
11. Purify using GenElute PCR Clean-Up Kit (NA1020). Elute in 50 µL nuclease-free water.
12. Determine the purified DNA’s concentration by measuring the absorbance at 260nm. One A260 unit is equivalent to 50ng/µL DNA. Yield at this point will vary depending on the quality of starting DNA, but is usually 1-5 µg.
Note: Alternative measurement techniques, such as PicoGreen®, will often underestimate the actual SeqPlex DNA yield.
At this point, the SeqPlex DNA is suitable for qPCR and microarrays. SeqPlex primer removal is recommended for deep sequencing.
Optional: Amplification quality may be assessed by gel or capillary electrophoresis. Typically, 1mL of crude or purified amplification product is sufficient for capillary chips, such as those for Agilent’s Bioanalyzer, while 5 mL is usually sufficient for agarose gel electrophoresis.
Primer Removal
Caution—Experienced GenomePlex WGA users:
SeqPlex Enhanced (SEQXE) Primer Removal reagents will not work on DNA amplified with previous generations of SeqPlex (SEQX) or GenomePlex (WGA1-5).
A reaction input of 2.1 μg is recommended to yield sufficient product for entering most deep sequencing workflows (>1μg).
13. Combine the following on ice:
8.0 µL - 10x Primer Removal Buffer (SR401)
1.6 µL - Primer Removal Solution (SR400)
X µL – 2.1 μg of purified SeqPlex DNA
Y µL - of Water (W4502)
--------------------------------------------------
76.25 µL Total reaction volume
14. Mix, and if performing the Optional Primer Removal Assay (see Appendix 1 below), remove 5 mL into a separate tube for “without primer removal enzyme” control.
15. Add 3.75 µL - Primer Removal Enzyme (SR402) to the remaining 71.25 µL from step 13.
16. Incubate both ± enzyme reactions as follows:
37 °C for 60 minutes
65 °C for 20 minutes
4 °C Hold
17. Remove reactions from the thermocycler, centrifuge briefly, and mix. Reactions may be stored at –20 °C for up to three days or purified immediately.
18. If performing the Optional Primer Removal Assay, remove and save 5 µL from the “with primer removal enzyme” reaction (see Appendix 1 below) prior to purification.
19. Purify the remaining reaction with primer removal enzyme using the GenElute PCR Clean-up Kit as described previously in step 10.
Sequencing
The SeqPlex DNA is now ready to enter Next-Generation Sequencing work flows, including end preparation, but generally does not require fragmentation or size selection. The bulk of SeqPlex DNA ranges from 200 to 500 base pairs. Though typically unnecessary, additional fragmentation can be accomplished mechanically or enzymatically.
Each SeqPlex amplicon terminus will have a 5’-phosphate and a 2-base 3’-over-hang after primer removal. Prior to ligation of sequencing primers, polish double-stranded fragment ends with T4 polymerase.
Any SeqPlex amplicons retaining primer after primer removal treatment will have a 5’-hydroxyl. T4 DNA kinase treatment is not required, and its omission will reduce ligation of sequencing primers to any residual undigested SeqPlex product.
The 3’-adenylation in Illumina’s workflow largely prevents ligation products, such as chimeras, from occurring. Chimeras have been reported with Roche 454 library preparation and sequencing. For ABI SoLID sequencing, a second sizing step prior to sequencing may help to reduce chimeras or concatemers.
Product Profile
This kit has been demonstrated to amplify ChIP DNA samples and whole genomic fragmented DNA templates. Note that a specific sequence within a highly complex DNA sample, such as ChIP input DNA, may not amplify to the same extent as that same sequence within a less complex DNA sample, such as ChIPed DNA. Therefore, representation levels are best compared between samples of similar complexity, such as ChIPed DNA from treated and untreated cells or tumor and normal tissue.
SeqPlex DNA has been successfully prepared for sequencing on Illumina’s GAIIx and MiSeq using standard Illumina workflow protocols including: end preparation, polishing, amplification and size selection.
We strongly recommend confirming the quality of SeqPlex DNA after primer removal by performing PCR with primers to short (<300bp) DNA sequences known to be present in the starting DNA before submitting samples for next generation sequencing.
Note: PCR products or primers containing the sequence 5’-CTGAAG-3’ or 5’-CTTCAG-3’ are not expected to amplify after primer removal.
Example of PCR product that WILL NOT amplify after primer removal:
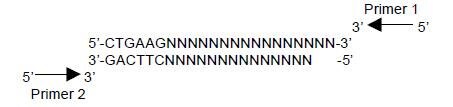
The presence of specific targets within SeqPlex DNA may also be confirmed by PCR or microarray before or after primer removal.
The stability of the SeqPlex DNA is equivalent to genomic DNA stored under identical conditions.
Appendix 1
Optional: Primer Removal Assay
The efficiency of primer removal can be assessed by performing qPCR using the 5X Amplification Mix (A5112), DNA Polymerase for SeqPlex (SP300), and the 5 µL retained samples from steps 18 &14 above for “with” and “without primer removal enzyme”. Sufficient 5X Amplification Mix and DNA Polymerase is provided to perform one Primer Removal Assay, “with” and “without primer removal enzyme”, for each amplification reaction.
- Serially dilute both “with” and “without primer removal” enzyme samples 1,000,000-fold (eg, 1 µL into 99 µL water, 3 times consecutively).
- Prepare assay mix for both by combining:
6.6 µL 5X Amplification Mix (A5112)
0.33 µL DNA Polymerase for SeqPlex (SP300)
0.33 µL 1/1,000 dilution, SYBR Green (S9430) in water
3.75 µL Water (W4502)
Note: Reagents requiring small volumes per assay may be diluted with water prior to addition to avoid measurement inaccuracies or an assay master mix may be prepared for multiple tests.
- Pipet 10 µL of both 1,000,000 fold diluted SeqPlex “with” and “without” enzyme into a separate PCR tubes or wells.
- Combine 5 µL assay mix from step 2 with each 10 µL of the 1,000,000 diluted SeqPlex products from step 3.
- Amplify as follows:
94° C for 2 minutes.
30 - 35 cycles*
94° C for 15 seconds
70° C for 5 minutes (read)
A Ct/Cq for the “with” Primer Removal Enzyme reaction that is at least 4 cycles greater than that for the “without” enzyme reaction indicates successful primer removal.
Reference
Frequently Asked Questions
- Is SeqPlex DNA compatible with microarrays and qPCR? Yes, SeqPlex DNA, with or without primer removal, may be used in these applications like genomic DNA or existing GenomePlex products.
- Are there advantages to SeqPlex over GenomePlex if primer removal is not needed? SeqPlex Pre-Amplification primers have been designed to target more frequently than existing GenomePlex WGA primers and therefore may provide the advantage of superior genome coverage in some regions.
- Will reducing cycles during amplification improve representation? No, Cycling at least 2-3 cycles beyond the "plateau" achieves optimum representation. Insufficient cycling leads to a significant reduction in representation/coverage.
- If monitoring amplification cycling via real-time PCR is not possible, how many cycles should be run? When optimum cycling conditions cannot be determined by monitoring, over-cycling is suggested. For 100 pg-1 ng ChIP samples; 24 cycles should be sufficient. For inputs less than 100 pg or poor quality; 29 cycles should be sufficient.
- Will the SeqPlex primer remaining after primer removal interfere with Next-Generation sequencer cluster calling? No, SeqPlex DNA with a minimal amount of primer remaining will generate excellent sequence on an Illumina GAIIx and MiSeq. To achieve optimum cluster calling of SeqPlex DNA, see Troubleshooting Guide for “NGS IVC abnormalities.”
- Will the SeqPlex Primer Removal Enzyme remove the entire SeqPlex primer? Yes, the SeqPlex Primer Removal Enzyme removes the complete primer, but the digestion may not be 100% complete.
- Will all copies of the SeqPlex primer be completely removed? A small amount of complete SeqPlex primer may remain. Most of the SeqPlex DNA (>90%) will have the SeqPlex primer completely removed.
- What yield should be expected? Yields are highly variable and dependent on a number of factors. Staring with 100 pg of ChIP DNA, roughly 1-5 ug of Amplification product and 1-3 µg of SeqPlex DNA ready for NGS library preparation is expected from each primer removal reaction.
Note: Up to 50% product reduction should be anticipated with primer removal. Therefore, if >1 μg is needed for sequencing, multiple separate 2 μg Primer Removal reactions should be performed.
- May the GenomePlex Reamplification kit (WGA3) be used with SeqPlex? No, the SeqPlex DNA before and after primer removal is incompatible with the GenomePlex reamplification kit. Primer sequences differ.
- Will SeqPlex DNA require special NGS sequencing protocols? No, SeqPlex DNA fits directly into NGS workflows, just like ChIP DNA. Sequencing instrument operators should be notified of running SeqPlex DNA and to expect a slight signal from any remaining primers in the IVC plots.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?