Performing a Purification of IgG Antibodies with MabSelect Sure
This section describes purification of monoclonal antibodies with HiTrap® MabSelect SuRe. The protocol includes a CIP procedure to minimize cross-contamination from different antibodies between purification runs, as well as for general column cleaning after a purification run.
Figure 1 shows capture of a human monoclonal antibody by affinity chromatography using HiTrap® MabSelect SuRe. SDS-PAGE of pooled, eluted fractions shows the high purity of the human MAb obtained in a single-step purification (SDS gel, lane 3).
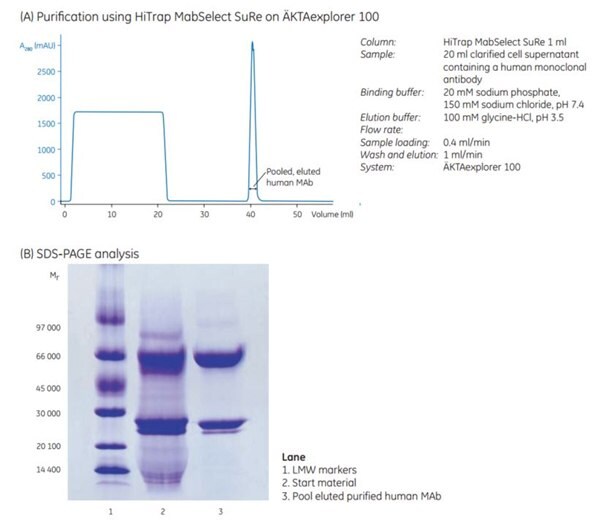
Figure 1.(A) One-step purification of a human monoclonal antibody on HiTrap® MabSelect SuRe 1 mL on AKTAexplorer 100. (B) SDS-PAGE analysis on ExcelGel SDS Gradient 8-18, reducing conditions.
Sample preparation
Refer to Desalting and Buffer Exchange for general considerations.
Centrifuge samples (10 000 × g for 10 min) to remove cells and debris. Filter through a 0.45 µM filter.
IgG from many species has a medium to strong affinity for protein A at physiological pH. Sample pH should be between 6 and 9 before applying to the column. If required, adjust sample conditions to the pH and ionic strength of the binding buffer by either buffer exchange on a desalting column or dilution and pH adjustment.
Buffer preparation
Binding buffer: 0.02 M sodium phosphate, 0.15 M sodium chloride, pH 7.2
Elution buffer: 0.1 M sodium citrate, pH 3.0 to 3.6
Neutralizing buffer: 1 M Tris-HCl, pH 9.0
Water and chemicals used for buffer preparation should be of high purity. Filter buffers through a 0.45 µM filter before use.
Purification
General instructions for purification using HiTrap® columns
- Prepare collection tubes by adding 60 to 200 µL of 1 M Tris-HCl, pH 9.0 per milliliter of fraction to be collected.
To preserve the activity of acid-labile IgG, we recommend adding 60 to 200 µL of 1 M Tris-HCl pH 9.0 to collection tubes, which ensures that the final pH of the sample will be approximately neutral.
- Fill the syringe or pump tubing with distilled water. Remove the stopper and connect the column to the syringe (use the connector supplied), laboratory pump, or chromatography system “drop to drop” to avoid introducing air into the system.
- Remove the snap-off end at the column outlet.
- Wash out the ethanol with 3 to 5 column volumes of distilled water.
- Equilibrate the column with at 10 column volumes of binding buffer. Recommended flow rates are 1 mL/min (1 mL column) and 5 mL/min (5 mL column)*.
- Apply the pretreated sample using a syringe fitted to the Luer connector or by pumping it onto the column. For optimal results, use a flow rate of 0.2 to 1 mL/min (1 mL column) and 0.5 to 5 mL/min (5 mL column) during sample application.
- Wash with binding buffer (generally at least 5 to 10 column volumes) until the absorbance reaches a steady baseline or no material remains in the effluent. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for washing.
- Elute with elution buffer using a one-step or linear gradient. For step elution, 5 column volumes is usually sufficient. For linear gradient elution, 10 to 20 column volumes are usually sufficient. Maintain a flow rate of 1 to 2 mL/min (1 mL column) and 5 to 10 mL/min (5 mL column) for elution. For purification using a syringe, elute with 2 to 5 column volumes of binding buffer.
* 1 mL/min corresponds to approximately 30 drops/min when using a syringe with a 1 mL HiTrap® column; 5 mL/min corresponds to approximately 120 drops/min when using a 5 mL HiTrap® column.
When optimizing elution conditions, determine the highest pH that allows efficient elution of antibody from the column. This will prevent denaturing sensitive antibodies due to exposure to low pH.
Stepwise elution allows the target antibody to be eluted in a more concentrated form, thus decreasing buffer consumption and shortening cycle times. It might be necessary to decrease the flow rate due to the high concentrations of protein in the eluted pool.
- If no cleaning-in-place is planned after elution, regenerate the column with 5 column volumes of elution buffer or and wash with 3 column volumes of binding buffer. If required, perform cleaning-in-place is directly after elution with at least 2 column volumes of 0.1 to 0.5 M sodium hydroxide ensuring a contact time of 10 to 15 min. Wash with 5 column volumes of binding buffer.
- Re-equilibrate the column with 5 to 10 column volumes binding buffer (or until the column has reached the same pH as the binding buffer).
Desalt and/or transfer purified IgG fractions to a suitable buffer using a desalting column (Desalting and buffer exchange).
Reuse of HiTrap® columns depends on the nature of the sample and should only be considered when processing identical samples to avoid cross-contamination.
Cleaning-in-place (CIP)
CIP can be performed to remove very tightly bound, precipitated, or denatured substances from the medium. If such contaminants are allowed to accumulate, they can affect the chromatographic properties of the column, reducing capacity and potentially contaminating subsequent purification runs. If the fouling is severe, it can block the column, increase back pressure, and reduce flow rate.
Regular CIP prevents the build-up of contaminants and helps to maintain the capacity, flow properties, and general performance of HiTrap® MabSelect SuRe. When an increase in backpressure is seen, the column should be cleaned. We recommend performing a blank run, including CIP, before the first purification is started to wash out possible trace amounts of leached protein A.
More information on CIP for HiTrap® MabSelect SuRe
Storage
Store in 20% ethanol at 2 °C to 8 °C.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?