Improving USP Lansoprazole Analysis with HPLC
USP Monographs
Compendial methods from the USP (United States Pharmacopeia) are widely used in pharmaceutical drug product and raw materials testing. However, not all methods in the USP use modern technologies. In chromatographic methods, it is not uncommon that older brands of columns are specified. Therefore, the USP methods are under continuous revision to improve existing procedures or to allow the user to obtain better results.
In an effort to improve the compliance of drug product, drug substance, and excipient monographs with current scientific/regulatory standards, USP is seeking the submission of proposals for improved methods. The intent is to replace the current procedures that may be deficient, flawed, or unsafe. Requests for revision of an existing monograph are encouraged by USP in light of advances in analytical technologies. Furthermore, ease of operation, suitability for automation, and potential for high-throughput analysis can be considered in a revision. To develop the best possible analytical test method for its intended use, a fully integrated method development process such as the selection of column, mobile phase, detection technology, and LC hardware by utilizing the most advanced technologies viable should be considered to ensure the methods are robust, consistent, and easy to use.
In this study, the USP method for lansoprazole was considered for improvement. Several drawbacks in the current USP monograph for lansoprazole prompted the investigation. These drawbacks include sample solution instability, use of different columns and samples preparations for the evaluation of assay and impurity, the requirement of using internal standard for assay and a long HPLC runtime (60 min). A new HPLC column, Ascentis® Express C18, based on Fused-Core particle technology was investigated for this study. The Ascentis® Express HPLC column claims high efficiencies as a result of a 0.5 µm layer of porous silica on a 1.7 µm solid silica core. An additional advantage to the column is that standard HPLC instrumentation can be used as opposed to UHPLC that is required for sub 2-µm columns.
Results and Discussion
Initially, a traditional 5 µm C18 column as specified in the USP monograph was compared to the 2.7 µm Ascentis® Express C18 using the standard USP conditions for chromatographic purity for lansoprazole (1,2). Improved resolution and sensitivity were obtained using the Fused-Core® column that allowed us to make several significant improvements to the method. Due to the improved resolving power of Fused-Core particles, the method was optimized with a shorter runtime without sacrificing resolution. The total run time was reduced from 60 min to 40 min (Table 1). Moreover, the improved sensitivity allowed for the reduction in concentration of the test sample for chromatographic purity from 250 mg/ mL to 100 mg/mL, the level required for assay in the USP monograph. Therefore, simultaneous evaluation of assay and chromatographic purity is achieved. Finally, a change of diluent pH, was implemented to improve sample solution stability removing the requirement of injecting sample within 10 minutes after preparation.
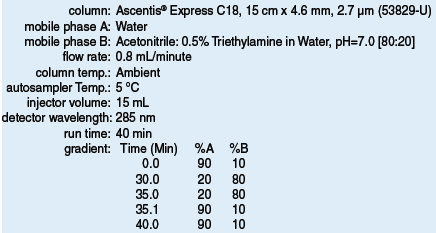
Table 1: Method Parameters for Improved Lansoprazole Method (53829-U)
The new method was shown to be linear from 0.05% to 150% of nominal concentration of 100 mg/mL, with quantitation limit less than 0.05%. The broad range of linearity allows for simultaneous impurity and assay analysis. The linearity data are shown in Figures 1 and 2. The RSD of 5 replicate injections of standard solution was 0.11%. In additional experiments, the method was evaluated by analysis of degraded lansoprazole drug substance. Lansoprazole was stressed under four separate conditions by exposure to acid, base, heat and hydrogen peroxide. The chromatograms of the acid and peroxide exposed drug substance along with the unstressed drug substance are shown for reference. The resolving power of the Ascentis® Express HPLC column makes it very suitable for these types of studies.
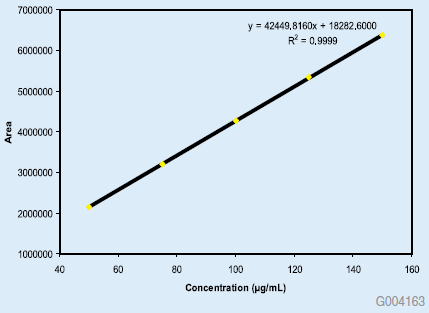
Figure 1: Linearity Curve from 50 to 150% Nominal Concentration
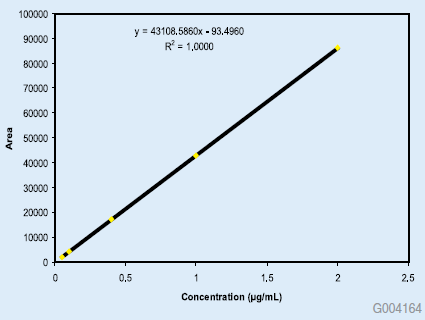
Figure 2:Linearity Curve from 0.05 to 2.0% Nominal Concentration
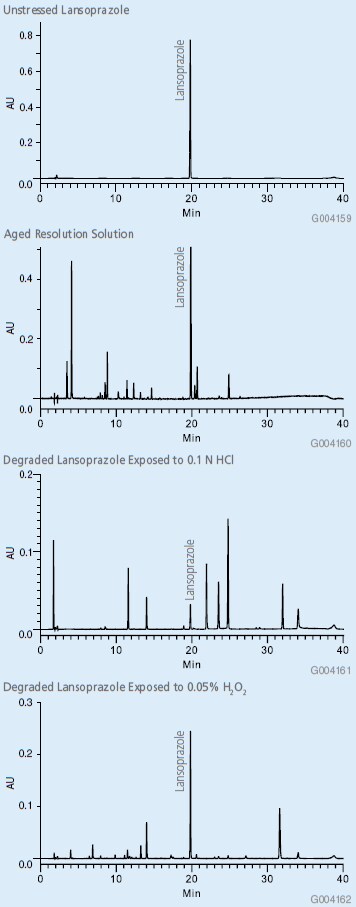
Figure 3: Analysis of Lansoprazole Using Improved Method with Ascentis® Express C18 HPLC Column
Conclusion
Improved resolution and sensitivity with a faster run time for Lansoprazole analysis
The method developed using 2.7 µm Ascentis® Express Fused-Core® C18 column provided significant improvements in comparison with the original USP method in terms of resolution, run time and sensitivity. As a result, the consolidated single method can be used for both assay and impurity quantitation. The advantages of Fused-Core columns as an alternative for sub-2 µm columns without using new UHPLC instruments could be appealing for pharmaceutical testing. Furthermore, this paper has presented one of the ways (a road map) that could be used by analytical scientists in the pharmaceutical field to improve USP monographs for their intended purposes using modern analytical technologies.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?