Validated Cell Types for Millicell® DCI Digital Cell Imager
Section Overview
The Millicell® DCI Digital Cell Imager enables quick, objective measurement of common cell culture parameters including confluency, cell count, and morphology across an extensive range of adherent and suspension cell cultures. Save time and conserve precious culture sample with in-vessel measurement. Track and record cell culture data using streamlined data management web tools. Analyze cell growth trends with instant access to historical data for more consistent cell cultures. Our R&D scientists have been working to validate the Millicell® DCI on some of the most common and some of the trickiest cell cultures. Select a category below to view cell lines and data.
Human Cell Lines
View data for validated human cells and cell lines including cell morphology, brightfield images at 10X and 20X magnification, confluency masks, growth curves, and more.
Non-human Cell Lines
View data for mouse, rat, and other non-human cell lines including cell morphology, brightfield images at 10X and 20X magnification, confluency masks, growth curves, and more.


Figure 1. Organoids before and after manual fragmentation. 3dGRO® patient-derived gastrointestinal organoids (SCC322) in dome culture were grown in L-WRN condition media (SCM105). After 6 days of proliferation (left), organoids were fragmented for passaging (right). Organoids were imaged directly using the Millicell® DCI under 10X magnification.
Cell cultures grown in Millicell® cell culture inserts
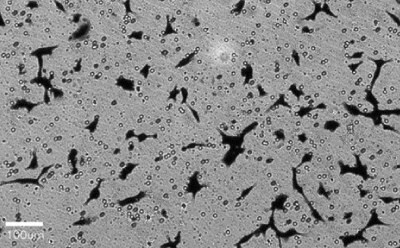
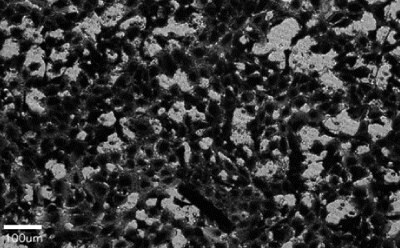
Figure 2. A cell migration and invasion assay was performed with HT1080 cells in Millicell® hanging inserts with 8.0 µm PET membrane (PTEP24H48). After 4-hour incubation in presence (left) or absence (right) of chemoattractant and fetal bovine serum, migrated cells were fixed and stained with crystal violet. The inserts were imaged directly using the Millicell® DCI under 20X magnification.
Suspension cell cultures
Suspension cell cultures can be more challenging to image and measure using digital cell imaging technology. We’ve validated the Millicell® DCI on suspension cell cultures, in addition to adherent cell cultures.


Figure 3. Jurkat E6.1 cells, a suspension cell line, were imaged using the Millicell® DCI under 10X magnification (left). Confluency was determined to be 22% by cell masking area (right).
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?