Workflow for Cannabinoids Analysis in Cannabis Using A Chromolith® Monolithic Silica HPLC Column Providing Low Backpressure and Extended Column Lifetime
Introduction: Analysis of Cannabinoids in Cannabis and Hemp Products
The legal use of recreational and medical cannabis is expanding globally along with hemp-based products (based on cannabidiol), for health and wellness. Hemp is defined legally in various geographies as cannabis varieties with limits on total tetrahydrocannabinol (THC) content. To ensure consumer safety, cannabis and hemp products need to be tested to determine accurate potency of the active cannabinoid constituents. Cannabis products in the market range from plants to distillates, and edibles to cosmetics. This broad variety of matrices underscores the need for robust columns and high throughput analytical methods.
This work provides a complete HPLC-DAD (high performance liquid chromatography-diode array detection) workflow for cannabinoids analysis using robust Chromolith® HighResolution (HR) HPLC columns which are based on monolithic silica. Chromolith® HPLC columns enable fast and cost-efficient separations due to low column backpressure and the very high robustness of the column. The low backpressure allows fast separation at high flow rates with the same mobile phase consumption per sample compared to slower low flow-rate methods. The workflow offers the following:
- Detailed hemp bud sample preparation for HPLC-UV analysis.
- Fast and cost-efficient separation with Chromolith® monolithic silica HPLC columns with low back pressure to determine potency of hemp bud sample.
- Demonstration of robustness of Chromolith® column.
- Separation of 14 major cannabinoids within 10 minutes.
- Calibration curve preparation using certified reference materials (CRMs) as cannabinoid standards.
Chemical Structure of 14 cannabinoids
There are more than 100 distinct cannabinoids that have been isolated from cannabis. Delta-9-Tetrahydrocannabinol (∆9-THC) is the primary psychoactive compound and cannabidiol (CBD) is another major non-psychoactive constituent in cannabis. Structures of ∆9-THC, CBD, and some other cannabinoids analyzed by the method described here are shown in Figure 1.
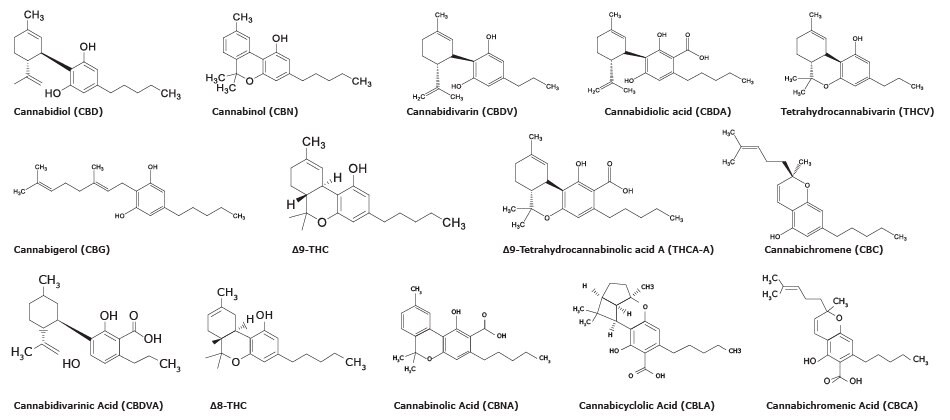
Figure 1.Chemical structures of fourteen cannabinoids included in this study.
Experimental Procedure: Cannabinoid Analysis in Hemp and Cannabis
In this work, hemp bud samples were analyzed to determine their potency. Sample preparation involved ethanol extraction of cannabinoids from plant material. The extract was then analyzed applying an HPLC-UV method and using a Chromolith® HR RP-18e monolithic silica HPLC column. Quantitation was performed utilizing a 6-point calibration curve obtained from HPLC-UV analysis of standard solutions prepared from CRMs. Peaks were identified using the retention times from a chromatogram of a 14 cannabinoids mix. Cannabinoid peaks were also verified by comparing UV absorption spectra of both samples and standards. Furthermore, robustness of the monolithic silica based Chromolith® was demonstrated via retention time stability and separation efficiency after 1400 injections.
Preparation of Mobile Phases
For mobile phase A, 0.1% H3PO4 (aq.) was prepared by adding 1 mL H3PO4 to 1000 mL of water. Pure methanol was used as mobile phase B.
Preparation of Standard Solutions
Standard solutions containing six major analytes were prepared using Supelco® CRMs as shown in the Table 1.
Preparation of Peak Identification Solutions
A peak identification solution containing 14 cannabinoids was prepared using CRMs, as shown in Table 2.
Extraction of Cannabinoids from Hemp Buds
Cannabinoids were extracted from hemp buds using ethanol extraction as explained below:
- Homogenize 1 g hemp bud (particle size <1 mm). (Low temperature homogenization such as frozen ball-milling is the preferred method of homogenization without sample degradation.1)
- Transfer the homogenized sample to a 50 mL polypropylene centrifuge tube.
- Dispense 20 mL ethanol and vortex for 30 s.
- Incubate sample on horizontal shaker for 30 min.
- Centrifuge sample at 4000 rpm for 5 min to pellet plant material.
- Transfer the supernatant into amber 100 mL volumetric flask and keep the pellet for second extraction.
- Perform second extraction with 20 mL ethanol and add the supernatant to amber 100 mL volumetric flask containing contents of the first extraction.
- Fill flask to 100 mL mark with ethanol and mix well.
- Perform 1:10 and 1:100 dilution of sample with ethanol.
- Filter samples into HPLC vials with 0.2 µm PTFE membrane. Here, syringeless filter-vials were used.
Subsequent analysis was performed applying a 2 mm I.D. Chromolith® HR RP-18e HPLC column using conditions described in Table 3.
Results and Discussion: Cannabinoids Separation with Chromolith® Column
Hemp bud sample was homogenized at low temperature to prevent analyte degradation using cryo-cup grinder followed by double extraction with ethanol. Resulting solution was diluted, filtered, and subjected to HPLC-DAD analysis. Calibration curves were obtained by analyzing solutions prepared from CRMs. Cannabinoids in hemp bud extract were identified based on retention time match with standards and cross verified with UV absorption spectra.
System Suitability - Peak Identification Solutions
CRMs as 1.0 mg/mL or 0.5 mg/mL solutions in methanol or acetonitrile, were used to prepare calibration and peak identification solutions.
Separation of 14 cannabinoids was demonstrated with good resolution and analyte signal reproducibility (Table 4). Separation of 14 cannabinoids was achieved in less than 10 minutes (Figure 2).
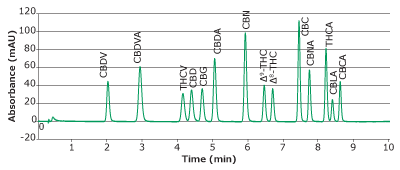
Figure 2.Chromatogram of 14 cannabinoids mixture obtained with a Chromolith® HR RP18e 50-2mm column at 228 nm.
Cannabinoid Quantification
Calibration curves were obtained for six major cannabinoids (CBD, CBG, CBDA, CBN, ∆9-THC, and THCA); see chromatogram in Figure 3. Linearity of R2>0.995 was obtained for all analytes within the range of 0.25-100 µg/mL (Figure 4).
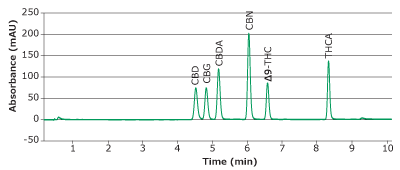
Figure 3.Chromatogram of calibration mixture at 100 µg/mL obtained with a Chromolith® HR RP18e 50-2mm column at 228 nm.
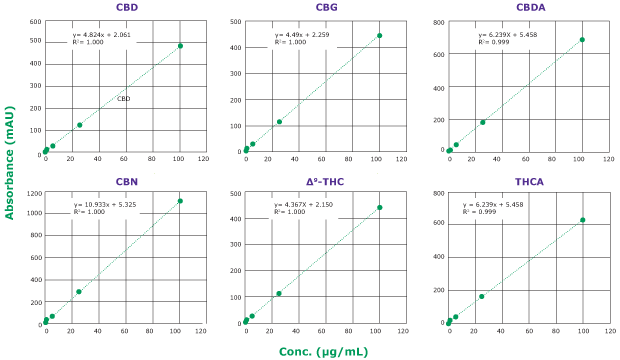
Figure 4.Calibration curves of six cannabinoid analytes obtained with a Chromolith® HR RP18e 50-2mm column at 228 nm. Calibration curve ranges from 0.25 to 100 µg/mL. Linearity: R2 >0.995 for all six analytes.
Results showed that the “as is” hemp bud sample contained 7.37% (wt/wt) total CBD and 0.25% (wt/ wt) total THC (Table 5). Stable retention time for cannabinoids was observed during 1400 injections, demonstrating the robustness of the column towards a complex matrix like hemp bud extract.
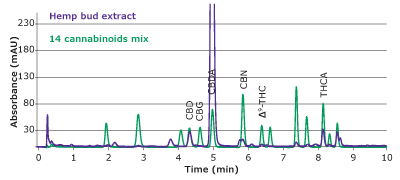
Figure 5.Overlay of chromatograms of peak identification solution and hemp bud extract obtained with a Chromolith® HR RP18e 50-2mm column at 228 nm.
In HPLC-UV analysis, identity of analytes depends on retention times and can be compromised by co-eluting peaks. Therefore, it is necessary to ensure that no co- elution of matrix compounds with the peak of interest is taking place. Here, we checked for the effects of matrix impurities by comparing the UV absorption spectra of the analytes identified in the sample with those of the standards. As can be seen in Figure 6, most analytes in the hemp bud extract display absorption profiles similar to those of the standard. Among them, the spectra of CBN seems to contain an impurity which is visible as an extra peak in the chromatogram in Figure 5 as well. This additional verification with UV absorption spectra further ensures the identity of detected analytes.
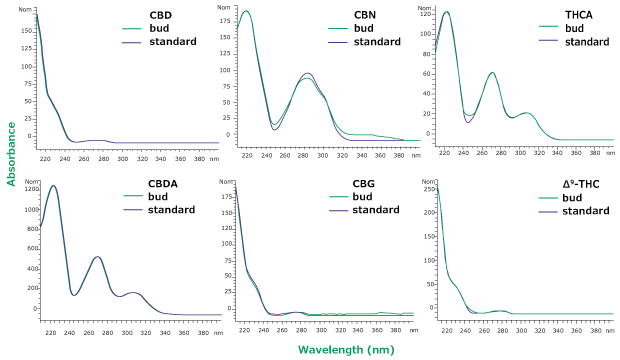
Figure 6.Overlay of UV spectra from hemp bud sample (green) and standard solution at same retention time (purple) for six analytes. Slight differences in CBN spectra between sample and standard might be due to the overlap with another peak as can be seen in Figure 5.
Robustness of Chromolith® Columns
The separation of matrix-rich samples, such as herbs, food, or biological samples tends to reduce the lifetime of particulate columns if insufficient sample preparation/cleanup is performed before HPLC separation. Chromolith® monolithic silica HPLC columns due to their bimodal pore structure allow the separation of matrix-rich samples with extended column lifetime, with no or very reduced sample preparation required. Extended column lifetime and reduced sample preparation significantly reduces the overall cost of operation. Figure 7 shows the stability of the retention factors for the analysis of cannabinoids in a hemp extract sample with a Chromolith® HR RP-18e 100×2 mm column over 1400 injections. Results demonstrate the robustness of monolithic silica based Chromolith® columns.
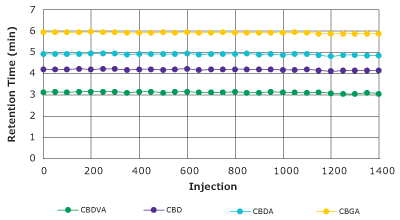
Figure 7.Retention factor stability performance test/hemp sample on a Chromolith® High Resolution RP-18e 100 x 2 mm (Column temperature of 25 °C, mobile phase A: 0.1% H3PO4, mobile phase B: methanol, flow rate 0.38 mL/min, injection volume 0.2 µL, gradient: 72% B for 0.1 min, 72-90% B in 7 min, hold at 90% B for 3 min).
In between cannabinoid sample analyses, the separation efficiency of the Chromolith® column was tracked with a performance test using anthracene. Separation efficiency was tracked by calculating plate count (N). Results were compared with those obtained for two other HPLC columns with small particle size: Ascentis® Express 2 µm (superficially porous particles, SPP) and Purospher® STAR 2 µm (fully porous particles, FPP) (Table 6). In addition, retention time stability was compared using CBD. For the CBD retention time comparison, the cannabinoid separation was optimized for the Chromolith® column and was then transferred to Purospher® STAR and Ascentis® Express columns in order to create comparable stability data. No significant change in the CBD retention time was observed after 1400 injections. The efficiency for the monolithic silica based Chromolith® column only slightly decreased (2.7%), while for the fully porous and superficially porous, a reduction of 25.3% and 11.3%, respectively, was determined. These results again show the robustness of both bimodal pore structure and rigid monolithic silica skeleton over an extended analysis period.
Conclusion
This work demonstrates an HPLC-DAD workflow, using a monolithic silica based Chromolith® HR RP-18e HPLC column, for the determination of cannabinoids in hemp bud samples. Sample homogenization, use of accurate CRMs, separation of 14 cannabinoids with good selectivity, and robustness of Chromolith® columns are important elements of the workflow. Hemp bud samples were homogenized at low temperature to prevent analyte degradation using a cryo-cup grinder, followed by double extraction with ethanol. The resulting solution was diluted, filtered, and subjected to HPLC-DAD analysis. Calibration curves were obtained by analyzing solutions prepared from CRMs. Cannabinoids in hemp bud extract were identified based on retention time match with standards and cross verified with UV absorption spectra. Results showed that the hemp bud samples contained 7.37% (wt/wt) total CBD and 0.25% (wt/wt) total THC on as is basis without determining dry weight data. The robustness of Chromolith® columns was also demonstrated by the analysis of hemp bud and stable retention factors for cannabinoids over 1400 injections, proving once more the suitability of these columns for matrix-rich samples.
Find more information on cannabis testing at SigmaAldrich.com/cannabis
Learn more about Chromolith® columns at SigmaAldrich.com/chromolith
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?