Mesoporous Oxides and Their Applications to Hydrogen Storage
Introduction
Solid-state hydrogen storage is attractive from a technological point of view, but has encountered tremendous challenges in terms of practical storage capacity and kinetics.1-3 Hydrogen sorption, whether it is chemisorption of dissociated atomic hydrogen or van der Waals weak physisorption of molecular hydrogen, depends strongly on material-specific surface interactions. High-surface area carbon materials and metal hydrides (including complex hydrides) represent two distinctive categories of candidate materials for solid-state hydrogen storage and have been the focus of intensive research.
More recently, metal-organic frameworks4,5 have emerged as an important class of physisorption hydrogen storage materials due to the possibility of designing them with functionalized porous structures as well as their low density and high specific surface area. On the other hand, reversible chemisorption materials, such as metal hydrides, exhibit high formula hydrogen storage capacities. However, their practical use is often limited by high thermodynamic stability (e.g., MgH2) or poor reaction kinetics (e.g., Alanates). New approaches that simultaneously address reaction chemistry, compositional modifications, and reactant heat and mass transport are needed to overcome these barriers to realizing practical hydrogen storage. To address these issues, we base our approach on low density, metal oxide based, ceramic materials produced using sol-gel processing, then modifying those structures using a variety of methods to form modified active oxide networks.
Low Density Mesoporous Oxides
Mesoporous materials, such as aerogels, offer several advantages over other materials due to their large surface area (over 1000 m2/gm), open porosity (80 to 99.9% porous), small pore sizes (typically peaking at 10-20 nm), and the ability to coat the surface of the mesoporous structure with one or more compounds. These materials are made using sol-gel processing followed by drying using solvent extraction. If the solvent extraction is carried out using supercritical processing the resultant material is called an aerogel. Aerogels were discovered by Kistler in 1931.6 Kistler’s pioneering work centered on demonstrating that a “gel” is made up of a solid framework containing the liquid from which it was formed. Because drying the gels in air causes them to shrink considerably and fracture into many pieces he surmised that surface tension forces were collapsing the submicroscopic pores. To avoid the surface tension forces he first substituted ethanol for the liquid in the gel, then raised the temperature and pressure above the critical point of ethanol (243.1°C and 63.1 bar) to eliminate the surface tension. At this point he slowly lowered the pressure, allowing the fluid to expand and leave the gel. He named the resulting material aerogel because it contained air rather than liquid. Alternatively, air dried gels are typically much denser and are termed xerogels.
Aerogels have been proposed for a wide variety of applications because of their unique properties. They are the lightest solid materials, with up to 99.7% porosity, transparent and excellent solid thermal insulators. In addition, aerogels have the lowest sound conductivity of any material (less than 100 m/sec, the sound velocity in air is 343 m/sec). They have been used to prepare Cherenkov counters, superinsulating windows, solar collector covers, refrigerators, spacecraft, water heaters and pipe insulation. Unique shock wave characteristics of ceramic aerogels led to their use as space dust collectors in earth orbit and the Stardust mission to the comet Wilde II which returned the first meteoric dust to earth. Aerogels have also been tested as high performance electrical insulation because of their high breakdown voltage, as catalysts due to their high surface area, for gas filters because of their fine pore sizes, for vapor phase pumps driven by temperature differences and aided by their hydrophilic nature, toxic cleanup materials, elements of acoustic devices and even safe insecticides.
An open pore aerogel structure may be made from many inorganic oxides, carbon, or polymers. In fact, aerogel mesoporous materials may be thought of as a “three dimensional canvas” that can be “painted” with a variety of materials on a nonporous scale. Great flexibility is afforded by this material’s processing technique because of the variety of backbone structures that can be produced and the ability to incorporate a large variety of additional constituents.7,8 This can be accomplished by modifying or adding functional groups to the sol gel precursor, or by chemical vapor infiltration (CVI) after the solvent has been extracted from the pores. CVI is chemically similar to chemical vapor deposition, CVD, but occurs inside a porous material. Using these techniques, it is possible to use substantial amounts of materials to coat the backbone with several times its volume. Thus, this approach to building mesoporous materials offers a flexible and cost effective route to a variety of materials tailored at the nanoscale for hydrogen storage as well as other applications.
We have been investigating the preparation and properties of ultra-low density nanomaterials based on aerogel technology since 1980. We also developed a technique to substitute nonflammable carbon dioxide for alcohol in the gel and perform the supercritical drying at lower temperatures (31.1°C and 73.0 bars).9 A wide variety of aerogel compositions have been prepared including metal oxides (SiO2, TiO2, Fe3O4, Al2O3, MgO, Cr2O3, Zr2O3), mixed oxides, and other compounds.10 Many of these have had additional coatings or deposits using pre- and post-supercritical drying treatments.11,12 A wide variety of elements and compounds have been deposited using sol-gel chemistry, solvent additions and Chemical Vapor Infiltration. The most widely investigated combination is silica aerogel coated with carbon using CVI with acetylene. Figure 1 illustrates a pure silica aerogel and a carbon doped silica aerogel. As a measure of the effectiveness of this process we have achieved from 1% to 400% loading of carbon into the silica (four times more carbon mass than silica).

Figure 1. Photographs showing un-doped (left) and carbon-doped (right) ultra-low density SiO2 network material.
Mesoporous Oxides for Hydrogen Storage
One of the key advantages of using ultralow density ceramic materials arises because of the size of the pores and the composition and functionality of the oxide backbone materials. Aerogels can be readily modified by changing the synthesis parameters. One modification approach is to vary the mixtures of precursors during preparation (the sol-gel stage). For instance, one can dope Si(OAlk)4 (679321, 679259, 679240, T5702, 333859) or Ti(OAlk)4 (Product No. 252670, 244112, 462551, 244759, 333484, 377996, 463582, 253081) with transition metal salts or boron compounds. During hydrolysis, transition metals can be incorporated into the oxide network. If functionalized dopants are used, such as A-CH2-Si(OAlk)3 (679267, 679356, 679291, 679275) or (A-CH2)2Si(OAlk)2 (371890, 435171, 539260, 446173), one can introduce additional chemisorption centers in the oxide network materials by coordinating A to transition metals (added after the network structure is formed). Additionally, the network material properties can be tuned by varying the compositions of the coatings, which would lead to applications far beyond hydrogen storage.
Once the mesoporous oxide networks are prepared, several approaches can be used to modify them. An example of the modification strategy, used to prepare X-SiO2 porous networks, is depicted in Figure 2, where X may be a high-capacity hydride compound, intercalated within the array of SiO2 nanoparticles of the aerogel, and filling a significant fraction of the pore space.
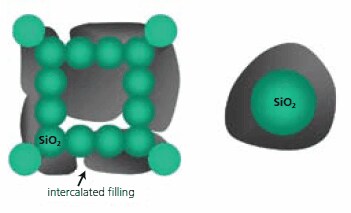
Figure 2.Schematic illustration of a strategy of modified active oxide networks. The right illustration represents individual hydride-X coated oxide nanostructure, forming an X-SiO2.
A schematic illustration (in two-dimensional projection plane) of the ultralow-density oxide network material is given in Figure 3a. It consists of a network of silica nanoparticles, approximately 3 nm in diameter, with a system of nanopores far smaller than the wavelength of visible light, typically 10 nm. Figure 3b is a transmission electron microscopy (TEM) image of a synthesized sample, illustrating that the highly porous medium consists of a network of randomly positioned silica nanoparticles. The specific surface area of the un-coated silica network is typically 1000 m2/g as measured by BET, and the bulk density is about 0.08 g/cm3.
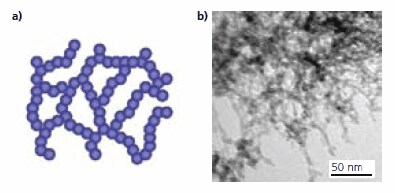
Figure 3. (a) Schematic illustration of a material consisting of a network of silica nanoparticles (b) TEM image of an ultralow-density silica oxide network sample.
We investigated the baseline properties of SiO2 ceramic nanoparticle networks for hydrogen storage to better understand and evaluate the effects of further modification of the materials. We found the SiO2 nanoparticle networks have favorable hydrogen storage properties through physisorption due to their very large surface area. Nevertheless, the storage capacity of the network does not yet meet the US DOE’s gravimetric and volumetric targets. The adsorbed capacity of an ultralow density silica network at liquid nitrogen temperature is shown in Figure 4. Measurements were conducted using a volumetric hydrogen storage testing system (PCTPro, Hy-Energy; see reference 13 for details). It can be seen that the hydrogen adsorption capacity increases monotonically to 40 bar and continues to rise with pressure.
Figure 4.Hydrogen storage capacity of ultralow density silicon network vs. pressure in bars.
To increase the density of stored hydrogen we introduce chemisorption into this system, while maintaining the benefit of the high-surface area for additional storage through functionalized physisorption. At the same time, the high-surface area nanoparticle network should provide the additional benefit of markedly improved chemisorption kinetics by increasing reaction surface area as well, as reducing diffusion distance (of hydrogen as well as constituent reactants).
As a proof of concept experiment, we fabricated a composite material using MgNi (<5 wt.%) implemented in an ultralow-density SiO2 aerogel nanoparticle network. Using magnesium, a structure was formed with 7.6 wt.% hydride, MgH2 (683043), but is generally hindered by poor kinetics caused by limited diffusion of hydrogen through the hydride. Ni is an effective catalyst for hydrogen dissociation and when combined with Mg may form the intermetallic compound Mg2Ni which also forms a hydrogen-rich complex hydride Mg2NiH4.
The composite network was prepared by a two-step process, starting with the pH-dependent hydrolysis and condensation of an alkoxysilane in alcohol, followed by CO2 substitution and supercritical drying. Under a high vacuum environment, the resulting SiO2 nanoparticle network was subjected to vapor infiltration of Mg and Ni through the use of a physical or a chemical vapor deposition process (we used a pulsed laser to vaporize a solid state MgNi alloy target). Preliminary measurements indicate chemisorption of hydrogen in the modified silica network, in addition to physisorption due to the very large surface area offered by the oxide network.
A technological advantage presented by the modified active oxide networks is their scalability. We have demonstrated large scale manufacturing feasibility of nanostructured oxide networks based on supercritical drying/sol-gel process and chemical vapor infiltration/deposition for incorporating desired compound through the mesopores of the oxide network. These ultralow density materials can be fabricated with varying density, structure and shape. Monolithic squares up to 20 inches on a side have been synthesized at Berkeley; they would be suitable for on-board transportation applications. With this scale-up manufacturing possibility, ultralow density active ceramic networks represent a clear alternative to existing porous media for solid-state hydrogen storage.
Summary
The above proof-of-concept experiments and analyses demonstrate the potential of the ultralow-density modified active oxide networks. While the hydrogen storage capacity of the mesoporous ceramic networks are not yet sufficient, it is our goal to modify them to achieve desired hydrogen storage capacity and kinetics. The optimized performance of the new materials can be realized by process modifications that include tuning the structure and specific surface area of the active oxide networks and implementing different combinations of catalytic and chemisorbing phases.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?