What is an Antibody-Drug Conjugate?
Antibody-drug conjugates (ADCs) are a fast-growing drug modality primarily used as a cancer therapeutics. As of early 2025, there are over a dozen ADCs approved by the FDA and around 300 ADCs in active clinical development.1, 2
This page describes what antibody drug conjugates are, their mode of action to kill a cancer cell, considerations to design and develop them, and how CDMOs can quickly bring them to clinic or market.
Section Overview
An ADC is typically composed of a highly potent active pharmaceutical ingredient (HPAPI), also called payload, attached to a monoclonal antibody core via a linker. This complex targets the drug to a specific cell type (e.g.: cancer cell) optimizing the therapeutic effect. In addition to these traditional operating modes, more recently developed ADCs also have the potential for improved efficacy through modulation of the patient's immune system.
Compared to traditional chemotherapy, ADCs have many advantages, including:
- Tumor selectivity
- Increased stability with longer half life
- Improved safety profiles (while still highly potent they exhibit reduced systemic toxicity)
Components of an Antibody-Drug Conjugate
The three primary components of an ADC: the antibody, the payload, and the linker are brought together by a bioconjugation (or conjugation) step, to create the ADC (Figure 1).
Monoclonal antibody: The antibody used in ADCs should be specific for a tumor-associated antigen that has restricted expression on normal cells. Most antibodies used are either humanized or human monoclonal antibodies.3 Antibody-derived material can also be used, such as antibody fragments (fAb), or bispecific antibodies, or completely different molecules including other proteins, radionuclides, oligonucleotide, nanoparticle or nucleic acids. This technical article will focus on ADCs developed from antibody-derivatives.
Payload: Payloads are commonly designed to kill target cells when internalized and released. When attached to a targeting antibody, these highly potent APIs can be delivered to cancer cells without negatively affecting healthy cells. Payload synthesis is a complex and multi-step process, requiring specialized production facilities for contained handling. Manufacturing can be streamlined and accelerated by using ADCore payload intermediates.
The payloads include diverse cytotoxic molecules including topoisomerase and tubulin inhibitors, DNA damaging agents, and immunomodulators. The family of tubulin polymerisation inhibitors (auristatins) is the most used followed by DNA damaging agents such as maytansinoids or camptothecins.
Linker: The linker attaches the payload to the antibody. It is designed to be stable in circulation and release the payload inside the targeted cells. Linkers can have many characteristics (e.g. cleavable vs. non-cleavable) and can affect ADC solubility, stability, toxicity. While first generation linkers lacked stability, second-generation linkers are designed for improved stability and include:
- Disulfide linkers (originally designed to be used with maytansinoids)
- Peptide linkers (most used peptide linkers and ideally suited to work with the auristatins)
- Thioether linkers
- Non-cleavable linkers (the activated drug retains the linker molecule and the lysine residue to which the linker was attached)
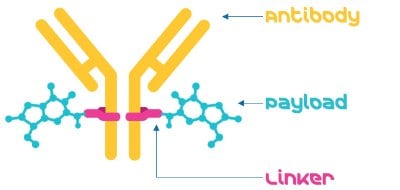
Figure 1.An Antibody-drug conjugate consists of an antibody, a linker, and a payload.
Bioconjugation typically yields a mix of ADC molecules, with heterogeneity from different locations and numbers of linker-payloads attaching to the mAb. This last element is characterized by the drug-to-antibody ratio (DAR): the average number of linker-payloads conjugated to an antibody. A low DAR can reduce the effectiveness of the ADC, and a high DAR can negatively impact antibody structure, stability, or binding and resulting safety profile. Site-specific methods and optimization of linker-payload to antibody ratio during bioconjugation reduces heterogeneity of the DAR and improves ADC consistency.
How do Antibody-Drug Conjugates work?
With advances in the field, the complexity of ADC modes of action and importance of tumor cell biology, is becoming clearer.4 The steps shown in Figure 2 provide a simplified, general representation of how these drugs work. For most ADCs with cytotoxic payloads, a cascade of steps leads to killing a cancer cell.
1. Binding: The antibody portion of the ADC binds the antigen on the cancer cell.
2. Uptake: The ADC is internalized into the cell and enters the endosome-lysosome pathway.
3. Antibody degradation: The ADC is degraded by the lysosome, separating the payload from the now degraded antibody and linker.
4. Payload release: The toxic payload is released from the lysosome into the cancer cell’s cytoplasm/nucleus.
5. DNA or microtubule disruption: Once released, the payload reaches its intercellular target.
6. Cell death: Cytotoxic effects of the payload result in the death of the cancer cell.
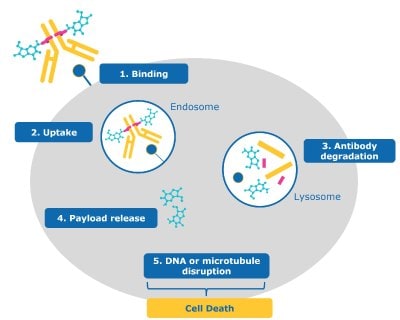
Figure 2.ADC mechanism of action for cancel cell targeting and killing (1) binding, (2) uptake, (3) antibody degradation, (4) payload release, (5) DNA or microtubule disruption, and (6) cell death.
ADC Design and Development Considerations
Because ADCs comprise multiple components that have varying effects on their efficacy, their design is complex. In addition to optimizing mAb, payload, and linker, bioconjugation includes additional considerations:
- Type of bioconjugation: Stochastic versus site-specific. Stochastic conjugation is the more traditional approach of (semi-)random modification while site-specific conjugation yields homogeneous products with a well-defined DAR
- Effects of bioconjugation on mAb: Changes to the mAb can affect its biological properties such as stability or targeting effectiveness. It’s important to consider these effects when choosing the linker and payload.
- Drug-to-antibody ratio optimization: A low DAR could reduce the antitumor efficacy, while high DAR may negatively affect antibody structure, stability, and antigen binding.
- ADC aggregation: Highly potent payloads, such as pyrrolobenzodiazepine (PBD) are hydrophobic and can cause aggregation. Addition of a solubilizer during discovery, formulation and/or concentration steps will resolve, or improve, ADC solubility and efficacy.
- Purification after bioconjugation reaction: Bioconjugation results in a mixture of ADCs with different DARs, free drug and linker molecules, and various solvents that need to be removed during purification.
- Chemical compatibility: In the development and manufacturing of ADCs, utilizing single-use technologies can improve process flexibility and reduce the risk of cross-contamination. However, it’s essential to verify that any single-use technology is chemically compatible with the organic solvents employed in ADC conjugation before implementation
ADC Contract Development and Manufacturing Considerations
Due to the complexities and containment handling requirements, many ADC developers select Contract Development, Manufacturing Organizations (CDMOs) to quickly bring their drug to clinic or market. These providers offer expertise in ADC manufacturing coupled with end-to-end services, testing capabilities, regulatory support and the know-how to seamlessly and safely expand from bench to production scales.
Learn more about products and services for ADC development and manufacturing.
The Future of ADCs and Next-generation Bioconjugates
The past years have increased our understanding on the complexities of ADC modes of action. A better understanding of the interplay between ADCs, the impact of their components, and cell biology, will support future innovations in ADC design to improve their efficiency, reduce toxicity and improve potential synergies with other drugs.
In parallel, while traditional ADCs use a mAb paired with a cytotoxic small molecule, next-generation bioconjugates have adopted the bioconjugate model for pairing various antibody formats (e.g.: fragment antigen-binding region (Fab region), single-chain variable fragments (scFv), and nanobodies) with diverse payloads (e.g.: small molecules, radionuclides, proteins, and oligonucleotides) (Figure 3). Such novel approaches to next generation bioconjugates open doors to innovative therapies to treat new indications.
Traditional
Figure 3a.mAb as component of an adc or bioconjugate
Figure 3b.Payload, or pan-cytotoxic small molecule as component of an adc or bioconjugate.
Emerging
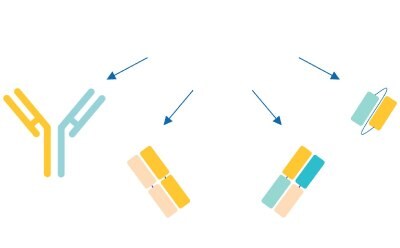
Figure 3c.Bispecific antibody and fragment-based bi-/multispecific constructs for payload delivery.
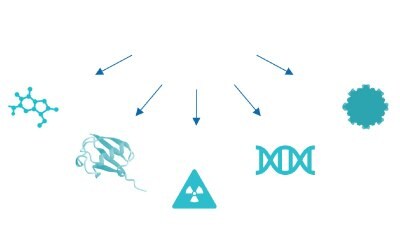
Figure 3d.Small molecule, protein, radio nuclide, oligonucleotide, nanoparticle.
Take the next step towards bringing your project to market quickly and safely by speaking to one of our technical experts.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?