Tangential Flow Filtration in the Antibody Drug Conjugate (ADC) Manufacturing Process
Tangential flow filtration (TFF) is essential in the manufacturing of antibody drug conjugates (ADCs), a type of drug where a cytotoxic drug is linked to a monoclonal antibody for targeted recognition. Ultrafiltration (UF) is used to concentrate the protein product and diafiltration (DF) to exchange buffer and/or remove process-related impurities from the ADC solution, including residual linker, organic solvent, and/or free drug (Figure 1).
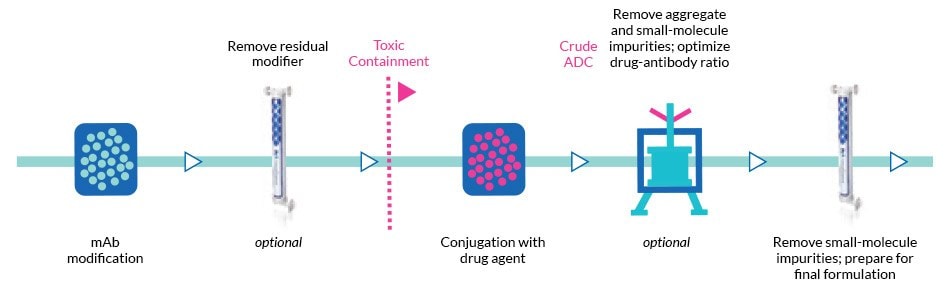
Figure 1.Example of an ADC manufacturing process.
Clearance of organic solvent is required when processing the ADC solution directly after conjugation, for which the TFF filter must be solvent-compatible. ADC manufacturing also requires a containment strategy during post-conjugation TFF steps to protect operators from health risks associated with exposure to the highly potent ADC molecule and free drug, all while preventing contamination of the product and the environment. As a result, self-contained, single-use TFF devices are a natural choice for UF/DF operations in ADC manufacturing (learn more about the various methods for tangential flow filtration here).
Below, we describe best practices for using our Pellicon® Capsules in ADC processing and share two studies using Pellicon® Capsules and Pellicon® cassettes in the ADC manufacturing process.
TFF Filter Organic Solvent Clearance and Compatibility
The ADC conjugation step occurs in the presence of an organic solvent, such as dimethyl sulfoxide (DMSO) or dimethylacetamide (DMAc). To process the crude ADC solution, the TFF filter must offer organic solvent compatibility with efficient clearance.
We used Pellicon® Capsules and Pellicon® 3 cassettes to demonstrate the clearance of 20% DMAc and 20% DMSO by diafiltration (Figures 2 and 3, respectively). Both filters performed with efficient solvent clearance and track a theoretical process in which no retention of the solvent by the membrane is assumed, calculated using a sieving coefficient (S) of 1. The decrease in DMSO removal rate after ~8 diavolumes may be due to the presence of dead legs in the system and charge interactions.
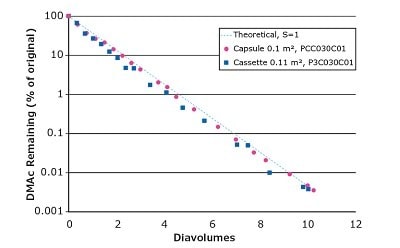
Figure 2.Diafiltration of 20% DMAc from aqueous solution.
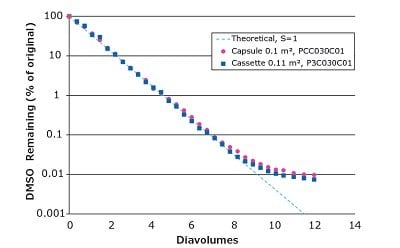
Figure 3.Diafiltration of 20% DMSO from aqueous solution.
Comparison of pre- and post-diafiltration parameters, including air diffusion, pressure drop, membrane permeability, and protein retention, indicates stability of the capsule to DMAc and DMSO under the tested conditions (Figures 4 and 5).

Figure 4.Compatibility of Pellicon® Capsule with DMAc.
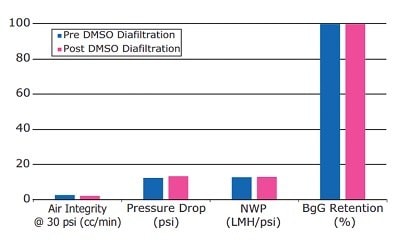
Figure 5.Compatibility of Pellicon® Capsule with DMSO.
Best Practices for ADC Processing with Pellicon® Capsule
When using Pellicon® Capsule for ADC feedstocks, the operating guidelines below should generally result in good process performance. These guidelines may be modified as needed based on findings from your process development work.
- Install Pellicon® Capsule(s) with 30 kDa Ultracel® membrane into the single-use TFF system for the filtration of modified mAb or ADC with molecular weight of ~150 kDa. Installation of the capsule does not require a compression holder or single-use liners/insert plates to contain the feed solution.1
- Confirm Pellicon® Capsule integrity, if desired.
- Condition the TFF system and membrane by flushing with 20 L/m2 of feed buffer (no sanitization is needed). After conditioning, prevent air from entering the capsule to avoid foam generation and protein damage from air/liquid interface.
- Add feed solution to the feed tank. If feed volume exceeds tank capacity, then continue addition in constant-volume fed-batch mode (feed flow rate into tank equal to permeate flow rate).
- Concentrate until the target concentration for diafiltration is achieved using a feed flow rate of 5 L/min/m2, 10-20 psi TMP (optimal TMP determined by running TMP excursions), at room temperature. For ADCs, typical target concentrations for diafiltration are within 25 to 30 g/L.
- Conduct diafiltration in constant-volume recirculation mode (buffer flow rate equal to permeate flow rate); this method typically gives the most efficient buffer exchange.
- Conduct final concentration. Over-concentrate as needed to compensate for any dilution expected during recovery and formulation steps.
- Recover the product from the Pellicon® Capsule and TFF system in a manner that gives desired yield, quality, and concentration. A reasonable recovery approach is to depolarize the membrane, drain the feed tank to a collection container, and then conduct buffer displacement (from the highest point of the system, down through the capsule) or buffer recirculation with one minimum working volume.2,3
- Remove the capsule and remainder of wetted flow path together from the single-use TFF system to keep the flow path closed, reducing risk of operator exposure to the process fluid.
- To scale up, increase area proportionally with feed volume, while maintaining the same normalized feed flow rate, TMP, and temperature.
ADC Mimic Case Study
Because of the toxicity associated with ADCs, ADC “mimics” are often used to study ADCs. These mimics are non-toxic and can be treated just as cytotoxic ADCs would be during conjugation and purification.
The applicability of Pellicon® Capsule in the processing of ADCs was showcased in a study reported by Czapkowski et al.4 A column-purified ADC mimic (~150 kDa) was used to evaluate and compare performance parameters of Pellicon® Capsule and Pellicon® 3 cassette with 30 kDa Ultracel® membrane. The ADC mimic solution was spiked with DMSO and diafiltered at a feed flow rate of 5 L/min/m2, TMP of 15 psi, and room temperature. The results indicated efficient clearance of DMSO from the ADC mimic feed solution by both capsule and cassette formats (Figure 6). In addition, permeate fluxes were stable throughout the diafiltration step for both filters, yields were high and comparable, and aggregate levels were also similar (Table 1).

Figure 6.Clearance of 6.8% DMSO by diafiltration of 26-28 g/L ADC mimic. Data from Czapkowski et al.4
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?