Optimizing ADC Purity and Yield Through Chromatography
Section Overview
- Expanding Demand for ADCs is Accompanied by Manufacturing Complexities
- Unique Purification Challenges of ADCs
- Mixed Mode Cation Exchange Technology for ADC Purification
- Optimization of ADC Purification Process Conditions
- Small-Scale Static Binding Studies to Optimize ADC Purification Conditions
- Dynamic Mode Evaluations of Chromatography Conditions
- Scale-up Considerations for ADC Purification
- The Future of ADC Chromatography: Efficiency and Cost Effectiveness
- Use of Eshmuno® CMX Mixed Mode Resin for Purification of Bispecific Antibodies
- Unlock ADC Purification Success
- Related Products
Expanding Demand for ADCs is Accompanied by Manufacturing Complexities
Antibody-drug conjugates (ADCs) are transforming cancer treatment by delivering highly potent therapies to targeted tumors with precision, thus minimizing systemic toxicity. With over 30 ADCs approved or filed for approval and more than 750 in development, the global market is rapidly expanding. However, the production of ADCs is accompanied by complex bioprocessing demands, especially in downstream purification. Ensuring a streamlined and robust purification workflow is critical to advancing ADC production and bringing more of these innovative therapies to patients.
For general information on ADCs and ADC manufacturing, visit this page.
Unique Purification Challenges of ADCs
ADCs present unique purification challenges that require specialized strategies to ensure product quality, yield, and safety. Among the requirements that the downstream purification process must address include:
- Heterogeneous DAR Distribution – During the production of ADCs, a variety of species with varying drug-to-antibody ratios (DARs) are generated. This increases the complexity of purification and requires advanced separation technologies with high selectivity to ensure the desired pharmacokinetics, efficacy, stability, and tolerability of the purified ADC.
- ADC Hydrophobicity – The hydrophobicity of many ADCs complicates purification using conventional chromatography methods, necessitating optimized resin selection and process conditions.
- Product-Related Impurities – High levels of aggregates, unconjugated antibodies and non-bound cytotoxic payload must be efficiently removed to meet purity standards.
- Yield vs. Purity Trade-off – Balancing high yield with optimal purity remains a fundamental challenge in ADC manufacturing.
- Safety Considerations – Since purification occurs post-conjugation, containment strategies are essential to protect operators from exposure to the highly potent cytotoxic ADC components.
Use of innovative purification approaches and tailored process development are critical to overcoming these hurdles and ensuring ADCs achieve their full therapeutic potential.
Mixed Mode Cation Exchange Technology for ADC Purification
Eshmuno®CMX is a mixed mode resin with 2-amino-4-methylpentanoic acid ligand that combines weak cation exchange with moderate hydrophobic interactions. It is particularly well-suited for the purification of hydrophobic ADCs and provides high-resolution separation of high and low DAR species while maintaining satisfactory recovery. As with all Eshmuno® ion exchange resins, the mixed mode resin combines a tentacle structure that can more effectively bind target molecules with the advantages of a rigid hydrophilic polyvinyl ether base matrix, enabling high flow rates and shorter processing times.
Figure 1 shows the UV and conductivity traces after applying a linear increasing pH gradient during separation of ADCs with varying DARs. Table 1 shows the analysis of collected fractions from the separation. The three peaks are DAR 2, DAR 4 and DAR 6 species (elution maximum at 98 mL, 104 mL, and 125 mL respectively).
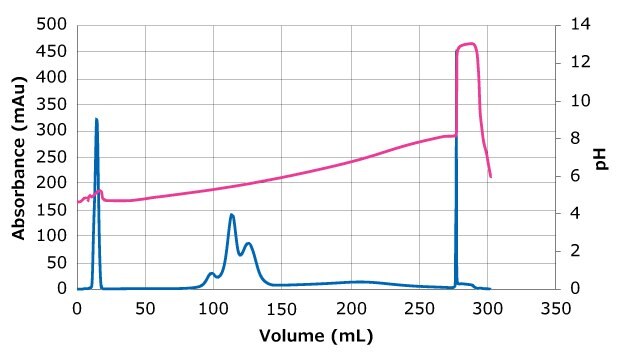
Figure 1.pH (yellow) and UV (blue) traces detected during chromatographic separation of ADC Adcetris® loaded to 10 mg/mL on Eshmuno® CMX resin, showing elution of sample within the pH change in three main peaks. The blue line represents protein concentration based on the UV adsorption.
Optimization of ADC Purification Process Conditions
The separation of DAR species using Eshmuno® CMX resin is typically performed in bind-and-elute mode at a pH of 4.5 to 7.5 with salt concentrations (e.g., Na₂SO₄) ranging from 0 to 1 M (Figure 2). Under these conditions, most ADCs bind to the resin, allowing for selective removal of low- and high-DAR antibody-drug conjugate species through optimized elution conditions. Variation in pH and conductivity increases the selectivity of the mixed mode cation exchange resin and can be performed in a gradient mode or step elution mode.
Small-Scale Static Binding Studies to Optimize ADC Purification Conditions
Small-scale static binding studies with the Eshmuno® CMX resin can be used to identify the optimal pH and conductivity conditions for effective separation. High-throughput screening (HTS) tools, such as 96-well plates or micro-columns, can accelerate evaluation while minimizing sample volumes, allowing exploration of broader operating windows. If HTS tools are unavailable, batch binding experiments can be manually conducted using small resin volumes in centrifuge tubes.
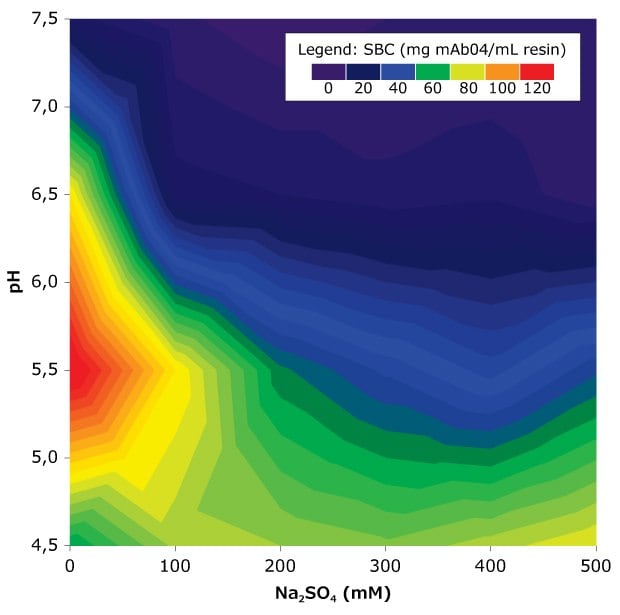
Figure 2.Quantitative example of antibody molecule binding to Eshmuno® CMX resin under a range of pH and conductivity conditions. A 50 mM acetate and 50 mM phosphate buffer system was used to achieve the pH with different Na2SO4 amounts. All values are average values from duplicates performed with 10 μL resin samples in microtiter plates. Static incubation was 120 minutes in 0.2 mL sample suspension.
Dynamic Mode Evaluations of Chromatography Conditions
While batch experiments can assess elution conditions, dynamic mode evaluations with a narrowed range of pH and conductivity in a column format are generally preferred to establish optimal binding conditions. This approach enables linear gradient elution and accounts for additional factors, such as protein loading, that impact ADC species resolution. The results from these preliminary screenings help refine the selection of resins and operating conditions for subsequent development stages.
A breakthrough curve for the ADC is generated to determine the dynamic binding capacity (DBC) of the Eshmuno® CMX resin at a specific breakthrough percentage: typically, 5% breakthrough is used for DBC determination with 3-5 minute residence times as a starting point. Studies should ideally be conducted using a column bed height that aligns with scale-up conditions (typically 15-25 cm). If feedstock is limited, a shorter bed height can be used for initial screening, with DBC later confirmed at the target bed height using the selected resin.
For complete details on optimizing Eshmuno® CMX for ADC purification, please refer to the application note: Antibody Drug Conjugate (ADC) Separation using a Mixed Mode Cation Exchange Chromatography Step in a Monoclonal Antibody (mAb) Process
Scale-up Considerations for ADC Purification
Several factors including column bed height, flow rate, and system pressure should be considered when scaling purification of ADCs from clinical to commercial production using Eshmuno® CMX resin. While these may be less important at small-scale, they become critical process parameters at commercial manufacturing scale. Buffer selection is also important, factoring in pKa, type, and compatibility with adjacent process steps. Common buffers include acetate, citrate, and phosphate, with cost, ease of use, and disposal implications considered at large scale.
For large-scale implementation, system and hardware capabilities must be assessed. Semi-rigid or rigid media, such as Eshmuno® CMX resin, have high permeability, maintaining pressure drops below 2 bar at linear velocities above 400 cm/h for a 20 cm bed height. Residence times for DBC evaluations should align with these constraints. Additionally, system pressure contributions from piping, column hardware, and tank head pressure must be factored along with resin bed pressure drop when designing large-scale processes.
The Future of ADC Chromatography: Efficiency and Cost Effectiveness
The future of ADC chromatography is focused on enhancing efficiency while reducing costs. By optimizing resin selection and purification strategies, ADC manufacturers can achieve higher yields, reduce buffer consumption, and minimize process steps, ultimately driving cost savings and increasing overall efficiency.
The integration of mixed-mode chromatography, such as Eshmuno® CMX resin, presents a significant opportunity to improve ADC purification workflows. With high selectivity, superior dynamic binding capacity, and improved recovery rates, this mixed mode resin contributes to enhanced performance, ensuring more efficient purification. Additionally, the resin's rigid base bead simplifies column packing, while its broad operational window and user-friendly process development make it an ideal choice for both early-stage and large-scale manufacturing.
Read more about the benefits of Eshumuno® CMX resin in Eshmuno® CMX Chromatography Resin: A highly selective mixed mode chromatography resin for difficult to purify mAbs, ADCs and fusion proteins.
Use of Eshmuno® CMX Mixed Mode Resin for Purification of Bispecific Antibodies
Eshmuno® CMX mixed mode resin can also be used to purify bispecific antibodies (bsAbs) which are hydrophobic, complex antibody formats. This mixed-mode resin demonstrated highly efficient impurity removal from an extremely hydrophobic bsAb in a single unit operation.1
While the resin achieves high purity (>97%), it significantly reduced product-related impurities and process-related host cell proteins (>3 log) while maintaining a satisfactory recovery of 70%. These results highlight the resin’s ability to purify highly hydrophobic antibody formats using moderate hydrophobic interactions.
With its high selectivity, broad operating range, and ability to eliminate the need for additional column purification steps, Eshmuno® CMX resin simplifies downstream processing and enhances the cost-effectiveness of complex antibody manufacturing.
Unlock ADC Purification Success
Optimize your ADC workflow with our chromatography systems and our Eshmuno® CMX mixed mode resin.
Related Products
Reference
To continue reading please sign in or create an account.
Don't Have An Account?