Addressing Viscosity Challenges for Subcutaneous Injections
Advantages of Subcutaneous Injections Versus Intravenous Administration
The majority of therapeutic antibodies, such as those used to treat cancer, are administered intravenously (IV). Most patients, however, would prefer the more convenient subcutaneous route. IV administration requires a visit to a healthcare facility, typically has a lengthy administration time, and is associated with higher costs and lower patient compliance. In contrast, a subcutaneous formulation can be given at the doctor’s office or a walk-in clinic, or even by self-injection in a home setting.
Challenges of Formulating Subcutaneous Injections
Subcutaneous Injections Require Small Volumes
There is however, a limit to the volume of drug that can be injected subcutaneously. Traditionally, subcutaneous formulations range from 1–2 mL, although volumes up to 3 mL are possible. On-body delivery devices with automated delivery via pumps allow larger volumes to be administered, but this route takes more time and can be inconvenient for the patient. With IV administration, the volume limitation does not exist; high volumes of drugs at a low concentration can be administered – but via a time-consuming process. The higher the dose required, the longer the patient receives the IV drip. In contrast, a bolus injection (> 5 ml) is not time consuming.
High Concentration Formulations Often Have High Viscosities
An obvious solution to the shortcomings of IV therapies is to produce higher-concentration versions, which offer the potential to reduce delivery volume and improve patient convenience and ultimately treatment success. For both originator biologics and biosimilars, this approach can translate into a real competitive advantage. But high formulation concentrations often result in higher viscosities. This viscosity is problematic for subcutaneous administration because viscous solutions don’t flow easily from the syringe through the needle and can cause pain at the injection site, or may not be able to be pushed through a syringe at all. The concentration-dependent viscosity challenge might limit drug manufacturers from even reaching the target concentration required for subcutaneous dosing during the manufacturing process due to pressure limitations.
At higher concentrations, proteins have greater opportunities to interact with one another to form transient clusters, which causes drug solutions to become more viscous. Typically, protein drug formulations below approximately 75 mg/mL are rarely viscous. At concentrations from 100–200 mg/mL, some proteins experience noticeable increase in viscosity, indicating a propensity for self-interaction, while others do not. At concentrations above 200 mg/mL, the prevalence of these self-interactions increases even without the existence of any specific affinity for one another. Figure 1 demonstrates concentration-dependent protein viscosity, either due to interactions or physical crowding, for several marketed antibodies.
Concentration Dependent Protein Viscosity
Concentration dependency of viscosity for market formulations
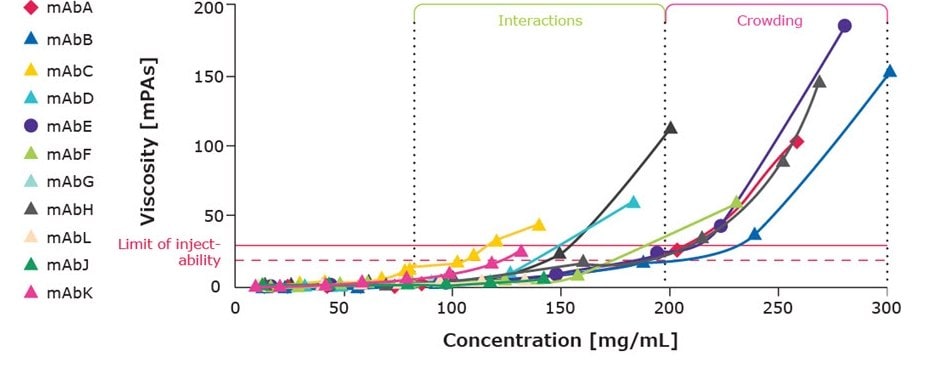
Figure 1.Concentration dependency of viscosity for marketed drug formulations.
Further complicating the matter is the fact that protein–protein interactions predominantly occur between the antigen binding fragment (Fab) regions, the same region that plays a key role in the efficacy of antibody drugs. Moreover, the forces involved in these interactions are responsible for stabilization of proteins via intramolecular interactions. This means any attempt to achieve lower viscosity creates a risk of reduced efficacy. Also, an antibody can become destabilized if attempts are made to reduce viscosity by interfering with protein–protein interactions.
Strategies for Formulating Subcutaneous Injections
One of the most common approaches to reducing viscosity is the use of a single excipient, typically arginine. Unfortunately, viscosity-reducing excipients do not always work across different proteins and antibodies. In addition, viscosity reduction is not always directly correlated to an increase in excipient concentration. Furthermore, increasing the excipient concentration may in some cases result in higher viscosity. It is also important to note that the use of excipients for viscosity reduction requires the formulator to balance this benefit with protein stability, which can put a limit on excipient concentration.
Separately, it is known that changes in pH — a critical parameter in biomolecule formulation development — lead to changes in the protein’s net charge. As such, the forces that influence the viscosity of protein formulations may be different at different pH levels. It should be expected, therefore, that different excipients may be required to formulate the same protein under different conditions.
Excipient Combinations from Different Functional Groups Address Viscosity Challenges
It was found that in contrast to single excipients, an amino acid and an anionic excipient can be more efficient at reducing viscosity when used in combination than when either is used alone, even at higher concentrations.
Selection of excipient combinations affords the flexibility needed to effectively balance viscosity reduction and protein stability for subcutaneous formulations.
Excipients can also perform synergistically, enabling improved viscosity reduction and a better balance of viscosity versus stability. Because it is possible to use lower concentrations of each excipient, negative impacts on protein stability are avoided.
The ability of a combination of an amino acid (ornithine, phenylalanine, arginine) and anionic excipient (benzenesulfonic acid, pyridoxine, thiamine phosphoric acid ester) to reduce viscosity and stabilize an antibody drug can be demonstrated based on data using two marketed drugs infliximab and evolocumab.
As shown in Figure 2, both drugs were concentrated up to 120 and 170 mg/mL respectively, which in the marketed formulations led to viscosity levels beyond injectability. For infliximab, adding arginine alone at 150 mM had only minimal impact on the solution viscosity. The various excipient combinations were added at 75 mM each, leading to multiple solutions below the injectability limit (25 mPa·s). The stability of these solutions was confirmed by long-term stability studies as shown in Figure 2. Both viscosity and stability using the single excipients were analyzed, confirming the superior performance of combinations (data not shown, available on request).
As each protein has its unique structure and characteristics, proteins tend to respond differently to formulation conditions including viscosity-reducing excipients. As such, there is no one-size-fits-all formulation; rather, a formulation toolbox is required which includes a choice of viscosity-reducing excipients. Evolocumab could be brought to an injectable level using the industry benchmark arginine. Still, multiple combinations were able to reduce the viscosity of the solution even further with the most efficient combination (Arg and TMP), not being the same as for infliximab (Phe and TMP).
Through synergistic effects, these combinations offered superior viscosity reduction, improved protein stability, storage stability, and better syringeability in comparison to the industry benchmark arginine and single excipients alone (data on single excipients not shown, available on request).
Superior Viscosity Reduction
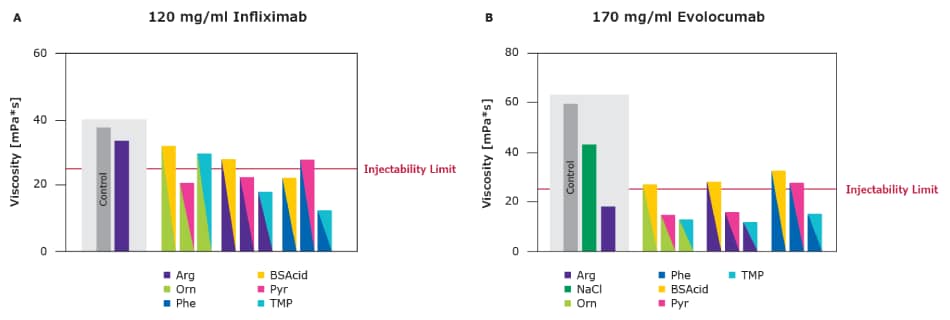
Figure 2.Excipient combinations offered superior viscosity reduction for 120 mg/mL Infliximab and 170 mg/mL Evolocumab in comparison to the industry benchmark arginine and single excipients.
Improved Stability
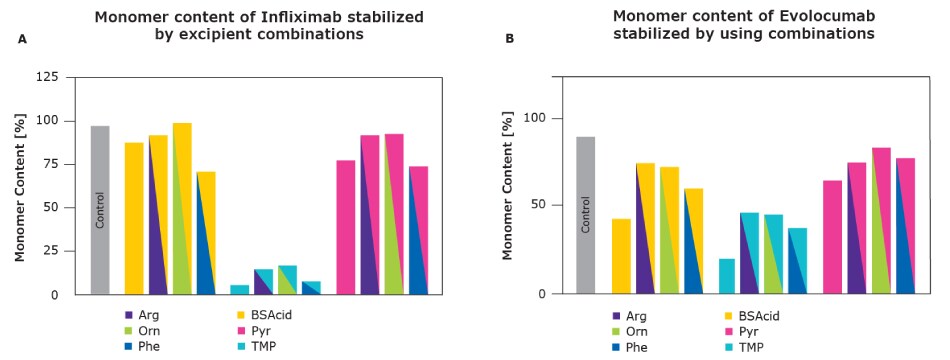
Figure 3.Excipient combinations delivered improved monomer stability for both Infliximab and Evolocumab in comparison to the industry benchmark arginine and single excipients.
Long Term Stability at Relevant Temperatures
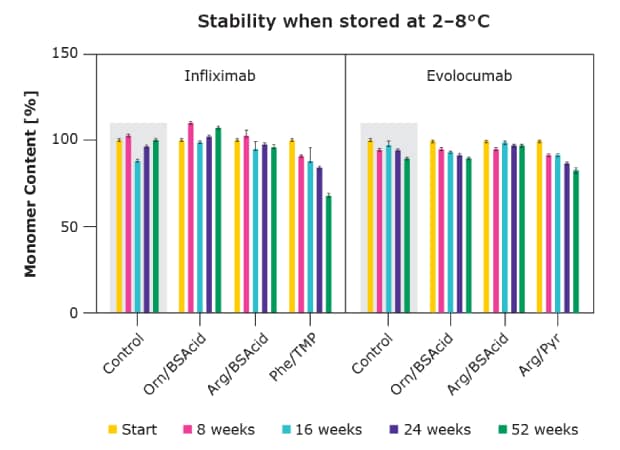
Figure 4.Long-term stability of Infliximab and Evolocumab using excipient combinations in comparison to the industry benchmark arginine and single excipients.
Improved Syringeability
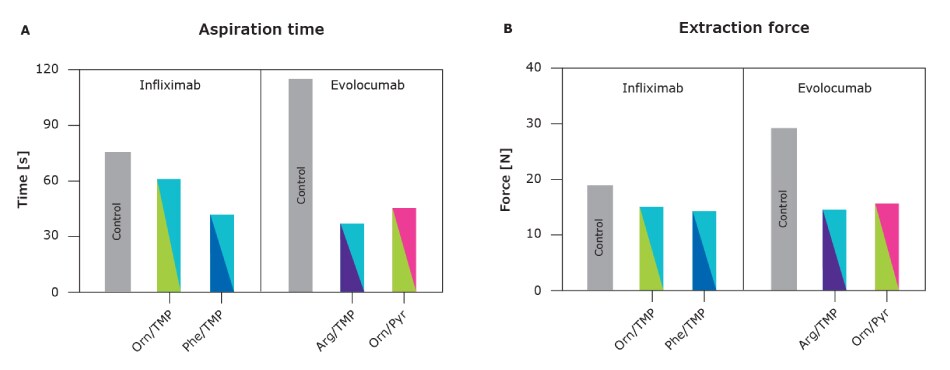
Figure 5.Improved syringeability as measured by aspiration time and injection force in comparison to the industry benchmark arginine and single excipients.
A Viscosity Reducing Toolbox
The SAFC® Viscosity Reduction Platform provides a toolbox of excipients that affords formulation scientists the ability to identify the right combination of excipients for reducing viscosity and maintaining protein stability for a given protein or antibody under specific formulation conditions. It is based on six components that are intended to be used in up to nine different combinations, always including one amino acid and one anionic component. The ability to vary these excipient combinations gives formulation scientists a high degree of flexibility for balancing formulation viscosity and protein stability, as well as helping to meet target pH levels and enable desired routes of administration.
For more detailed information on reducing the viscosity of high concentration drug formulations to enable subcutaneous injections:
- Download the white paper Viscosity-reducing excipients for protein formulation.
- Watch the on-demand webinar The Viscosity Reduction Platform - Enabling Subcutaneous (subQ) Delivery.
Related Videos
Materials
To continue reading please sign in or create an account.
Don't Have An Account?