Developing and Manufacturing Attenuated Viral Vaccines
- How Viruses Become Attenuated
- Cell-based Viral Vaccine Manufacturing
- Cell-based Propagation of Attenuated Viruses
- Clarification of Attenuated Viruses
- Nuclease Treatment
- Chromatography
- Sterile Filtration, Formulation, and Fill Finish of Attenuated Viral Vaccines
- Related Products
- References
Attenuated viral vaccines are created using viruses that have reduced virulence. They offer quick immunity, activate all phases of the immune system, and provide durable long-term immunity.
How Viruses Become Attenuated
Viruses are generally attenuated via passage in unrelated or foreign hosts such as tissue culture, embryonated eggs, or live animals. During passage, mutations that arise can enable the virus to infect a new host. Through a series of passages, the virus can acquire mutations that make it less virulent in the original host. This version can be used for a vaccine.
As an alternative to mutations through passage, attenuated viruses can be created using genetic engineering to precisely introduce mutations, gene deletions, or substitutions.
However, there are some drawbacks in using attenuated vaccines. A secondary mutation in the attenuated virus can cause a reversion to virulence. This means the vaccine may be able to cause disease in immunocompromised patients (those with AIDS, for example). Additionally, they can be difficult to transport because they must be maintained under certain conditions, such as a low temperature, to guarantee the survival of the virus.
Cell-based Viral Vaccine Manufacturing
Live attenuated viral vaccines can be created using a complex, multi-step cell-based manufacturing process. It is not a templated process. The manufacturing process for each viral vaccine is different and is dictated by shape, size, physico-chemical behavior, stability, and host specificity. Though different manufacturers follow different process flows, a general outline of the process is summarized in Figure 1. An important manufacturing challenge in developing attenuated viral vaccines is that the virus must be kept live and maintain its infective potential throughout downstream processing and formulation until it is administered to healthy individuals.
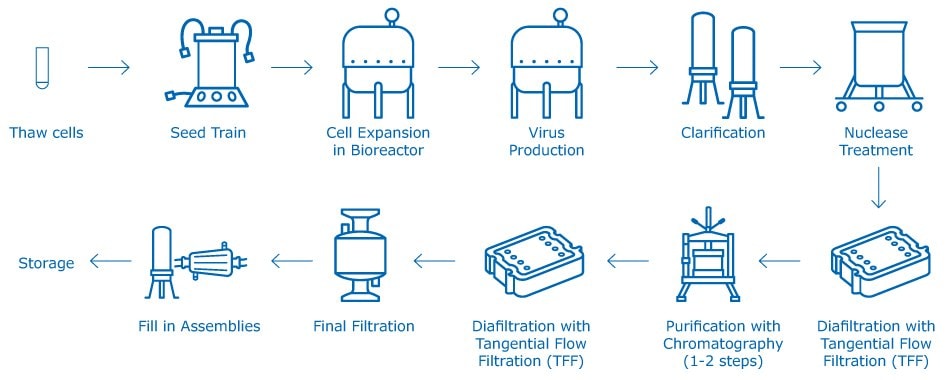
Figure 1.Generic attenuated viral vaccine manufacturing process.
Cell-based Propagation of Attenuated Viruses
Viruses are propagated in cell culture, grown either in roller bottles (as a monolayer) or suspension cultures, or bound to microcarriers. A typical pooled roller bottle batch volume is 500–700 L, and suspension culture is 1,000–2,000 L.
There are several types of cells used for growing viruses for vaccine application:
- Vero
- Per.C6
- MDCK
- MRC 5
- WI38
Vero cells (developed from African green monkey kidney cells) are most commonly used for viral vaccine manufacturing. Most cell cultures for viral vaccine applications are grown in low-oxygen tension in the presence of ~5% CO2. The cell culture is grown for five to seven days prior to viral inoculation. Virus harvesting occurs after 24 to 72 hours of inoculation. Depending on the virus type, they either bud out of cells or lyse the cells and emerge in the extracellular culture fluid. In some cases, the cells need to be lysed by the addition of detergents or surfactants (for example, Tween® 20 nonionic detergent) to release the viruses.
Clarification of Attenuated Viruses
Clarification removes the cells or cell debris and harvests viruses. Zonal centrifugation is commonly used for primary clarification. Some manufacturers also use tangential flow filtration (TFF) under low shear conditions or normal flow filtration (NFF), in most case depth filtration, for clarification of viral vaccine.
Attenuated viruses are fragile and shear sensitive. Microfiltration (MF) TFF devices (without screen) are preferred to minimize shear. Solid content in viral vaccine harvest is low, so normal flow filters also work well for such applications. Some attenuated live viruses tend to bind to cell surfaces or get trapped in lysed cell debris. This leads to their removal during clarification resulting in poor virus recovery. Because viruses are negatively charged, it is important to be aware of adsorptive effects on filter media.
Nuclease Treatment
Nucleic acids are negatively charged large molecular components that interfere in virus purification. Carryover nucleic acid from lysed cells is a key contaminant in viral vaccine processes. Viruses propagated in human diploid cells or non-human cells (for example, viruses grown in dog kidney cell lines [MDCK]) pose a greater risk of nucleic acid carryover. Regulations require that carryover host cell nucleic acid content should be below 10 ng/dose of attenuated viral vaccine.
Benzonase® endonuclease is commonly used to degrade the nucleic acids such as RNA and DNA of the host cells to as low as three to eight base pairs (<6 kDa). The virus harvest is treated with ~0.9 to ~1.1 units/mL of Benzonase® endonuclease at 30–34 °C for four to eight hours. After Benzonase® endonuclease treatments, the harvest is diafiltered using TFF (100–300 kDa ultrafiltration devices) operating at low crossflow to remove Benzonase® endonuclease and degraded nucleic acid components. The typical flux (for 300 kDa Biomax® ultrafiltration membrane) is 25 LMH at 1.5–3.0 psi transmembrane pressure (TMP) and 4-5 L/min/m² feed flow rate.
Chromatography
Benzonase® endonuclease treatment is sufficient to bring most attenuated viral vaccines—measles, mumps, rubella, polio, rotavirus, and yellow fever among others—to the desired level of purity during the concentration and diafiltration step. However, chromatography is normally required to bring new generation of viral vaccines like Japanese encephalitis virus (JEV) and dengue virus (DENV) to the desired level of purity. For example, sulfate ester covalently linked to a cellulose matrix can be used to purify JEV. The virus binds to matrix based on mixed-mode interaction with virus surface receptors or heparin-binding domain present on a few enveloped viruses. As viruses are negatively charged, anion exchange chromatography (Q or DEAE) works well in bind and elute mode or flow through mode. These operations run in mild conditions with low salt.
Post chromatography, the eluted virus is concentrated by using 100–300 kDa TFF devices. Purity of the live viral vaccines is determined by measuring the removed contaminants (bovine serum albumin, ovalbumin, residual DNA, host cell protein, etc). Quality and quantity of virus in the purified bulk is determined by estimation of virus concentration based on HA titer, neutralizing antibodies, and CCID50 infectivity assay.
Sterile Filtration, Formulation, and Fill Finish of Attenuated Viral Vaccines
Final virus vaccine bulk is comparable to that of water. During final filtration, the vaccine is filter sterilized using 0.22 µm sterilizing filtration.
There are special considerations in formulating attenuated viral vaccines:
Multivalency
Many of the attenuated viral vaccines are formulated with different strains. These multivalent vaccines include rotavirus, polio, and dengue. They are aseptically blended after sterile filtration.
Adjuvants
Most of the live attenuated viral vaccines do not need any adjuvants because they are naturally potent immunogens. Most of them, for example, measles, mumps, and rubella, are lyophilized (freeze-dried).
Excipients
The final formulation of attenuated viral vaccines contains a small amount of antibiotics (neomycin), excipient (human serum albumin, HAS), stabilizer (hydrolyzed gelatin, egg protein, sorbitol, sucrose) and buffering agents (NaCl, other salts). Most of these vaccines are administered subcutaneously except for rotavirus and polio virus vaccines, which are isotonic solutions that are administered orally.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?