Reproducibility with Biological Buffers
What Are Biological Buffers?
Biological buffers are organic substances that maintain a constant pH over a given range by neutralizing the effects of hydrogen ions. Buffers are commonly used in biochemical, biological and environmental studies to maintain or control the acidity of a solution within a desired physiological range, or even initiate a particular reaction. The pH of substances not only affects the rate and efficiency of chemical reactions, but also impacts the recovery and purity of products. In biological studies, the pH has an effect on the structure and function of proteins, enzymatic reactions and cellular metabolism. Therefore, buffers significantly contribute to the outcome of such studies. The most widely used buffers in biological studies are zwitterionic N-substituted amino acid derivatives, also referred to as biological buffers or Good’s buffers.1,2
Criteria for Biological Buffers
Although none of the biological buffers fulfills all of the requirements below, they are expected to satisfy the majority of them:
- pKa between 6.0 and 8.0, a range where most biological reactions occur
- High water solubility and low organic solvent solubility, the ratio of which determines the distribution of the buffer between the aqueous medium and the biological phase
- Low lipid solubility with biological membrane impermeability
- Minimal effect on the dissociation of the buffer from any changes to temperature, ionic strength and concentration
- Preferably does not form complexes with trace metals, alkali metals or other ions
- High stability with resistance to enzymatic degradation; does not act as enzyme inhibitors or substrate analogs
- Does not absorb light in the visible or UV spectrum that are mainly used in spectrophotometric assays
- Minimal interactions with salt and critical reaction constituents
- Cost-effective and easy to prepare
Low Reproducibility Rate of Preclinical Research
Reproducibility refers to the ability to produce the same results in research experiments using the exact same materials and procedures. Reproducing the original results of published discoveries is crucial for the biomedical research community in order to make conclusions and explore new areas of research, which can translate into therapeutic applications. Ultimately, such lost time and opportunities negatively impact the discovery of life-saving therapies and cures.
However, reproducible research has long been a major challenge in the scientific community, as many researchers rely on existing basic research information to conduct further studies, which may not be reliable and can lead to faulty assumptions and significant losses of time and money. Leonard P. Freedman, President of the Global Biological Standards Institute, and co-authors published a paper “The Economics of Reproducibility in Preclinical Research” in 2015 that estimates a shocking $28 billion are spent each year on preclinical medical research that cannot be reproduced.
Research reproducibility failure and follow-on research based on flawed premises not only wastes resources and funds it may also affect the reputation and career prospects of researchers and research institutes, and may have a negative impact on the journal reputation and readership.
Several recent publications have highlighted the issue of low reproducibility rate in preclinical research. In 2012, scientists from Amgen reported that they were only able to reproduce 6 out of 53 ‘landmark’ preclinical studies, which resulted in more discussions among the scientific community regarding the scale of the reproducibility problem and the development of initiatives to overcome this problem.
In general, irreproducibility can be caused by study design, the inaccuracy of the laboratory protocol, poor data analysis and reporting, and the unsuitability of biological reagents and reference materials used.3 The image below illustrates the key sources of irreproducibility and the magnitude of this crisis.
Sources of Irreproducibility - The Cost is High
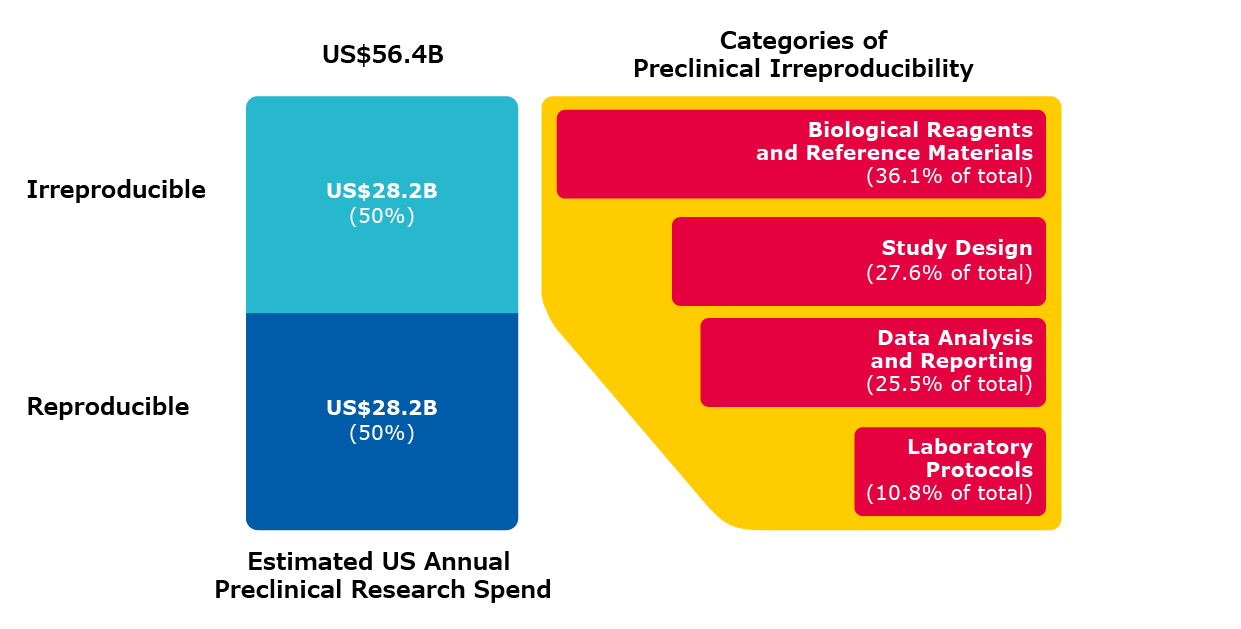
Figure 1. Estimated US preclinical research spend and categories of errors that contribute to irreproducibility. [Note that the percentage value of error for each category is the midpoint of the high and low prevalence estimates for that category divided (weighted) by the sum of all midpoint error rates]
The “Second Annual State of Translational Research” survey was conducted in 2014, where participants answered questions about which product groups caused the most difficulties with research reproducibility. The results of this survey revealed that animal models, antibodies, cell lines, PCR reagents and primers, cell culture reagents, fluorescent tags, dyes, buffers and solvents were of major concern.
Buffers are widely used in a range of protocols and applications in basic research, industrial pharmaceutical and biopharmaceutical markets. Variability in the selection and quality of these buffers can have a significant impact on research reproducibility. Therefore, there is a great need for better understanding and training on their selection and use.
Biological Buffers Selection Guide
Most biological processes are pH-dependent and any minor change in pH can lead to variation in the biological function, biomolecule stability and the outcome of the experiment. The main purpose of a buffer in a biological system is to resist change in pH due to internal and external influences and maintain intracellular and extracellular pH within a narrow range.
Potential obstacles with buffers can be classified into two categories:
a. Lot-to-lot variability with buffer
- Water content: Lot-to-lot variability can change the solution concentration if buffers are weighed and not corrected for water content.
- Morphology: Buffer components are crystalline products with variations in particle size, particle form, milling characteristics and packing density. This can have an impact on the infrared (IR) scan. For example, HEPES is polymorphic and can exist in 2-3 different morphologies; accordingly, its IR scan may vary. Therefore, it is important to choose a suitable buffer if an IR scan is used as a characterization criterion.
- Trace elements: The presence of trace elements can have an impact on the biological systems and cell culture. For example, the presence of Cu, Zn and Se is critical in the performance of mammalian cell culture during antibody production and can affect the yield of therapeutic antibodies.
- Impurities from synthesis: Byproducts and artifacts, such as polyvinyl sulfonic acid (PVS) and free amines, that are produced during the synthesis of many organic buffers can impact the performance of biological processes including enzymes activity, protein aggregation and bioactive properties.
High purity of buffers is critical to assure minimal levels of trace elements, chemical impurities and biological contaminants that provide lot-to-lot consistency for better reproducibility.
b. Interactions with biological systems
Although biological buffers are assumed to have minimal interaction with the metal ions present in environmental and biological systems, several studies have recently indicated otherwise. Therefore, it is essential to comprehend how these buffers interact with metal ions and other biological materials, such as proteins.
For example, any enzyme that requires metal ions for its activity may be inhibited if the buffer that is being used forms an insoluble complex with those metal ions. Complexes should, therefore, be soluble and their binding constant known. Phosphates form insoluble salts with bivalent metals and precipitate. Therefore, a phosphate buffered salt (PBS) solution is never autoclaved with Ca2+ or Mg2+. Biological buffers, such as PIPES, TES, HEPES and CAPS, have very low metal-binding constants and are particularly suited to investigate metal-dependent enzymes. However, Bis-Tris Propane, ADA and Tris all interact strongly with various metal ions.
Below is an overview of complexation magnitude strength among the different metal-buffer pairs2 :
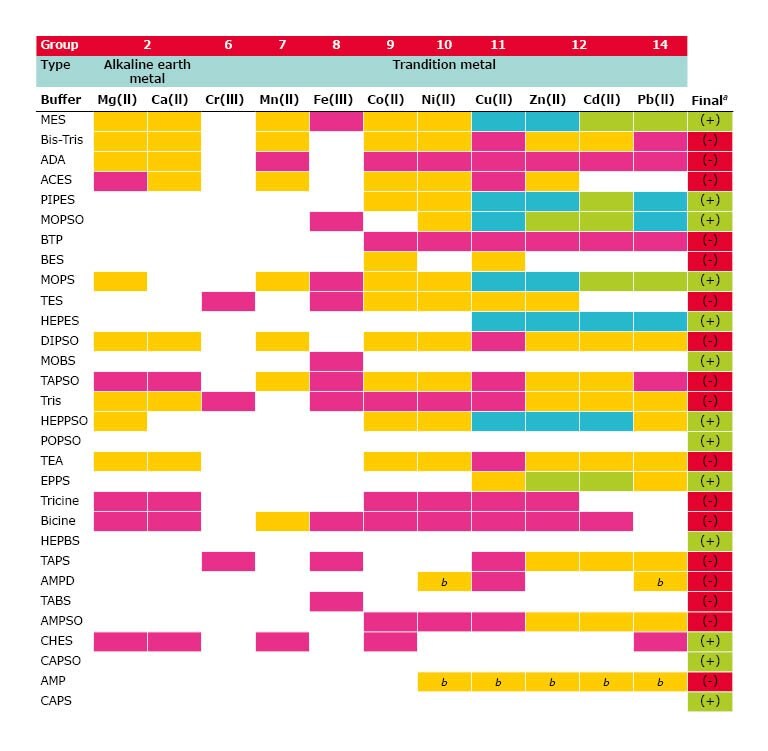
Figure 2. Metal-Buffer Pairs - Complexation
- Green = No complexation, suitable for general use
- Yellow = Light complexation
- Magenta = Strong complexation, not suitable for general use
- Cyan = Data not in agreement (Further studies or caution necessary)
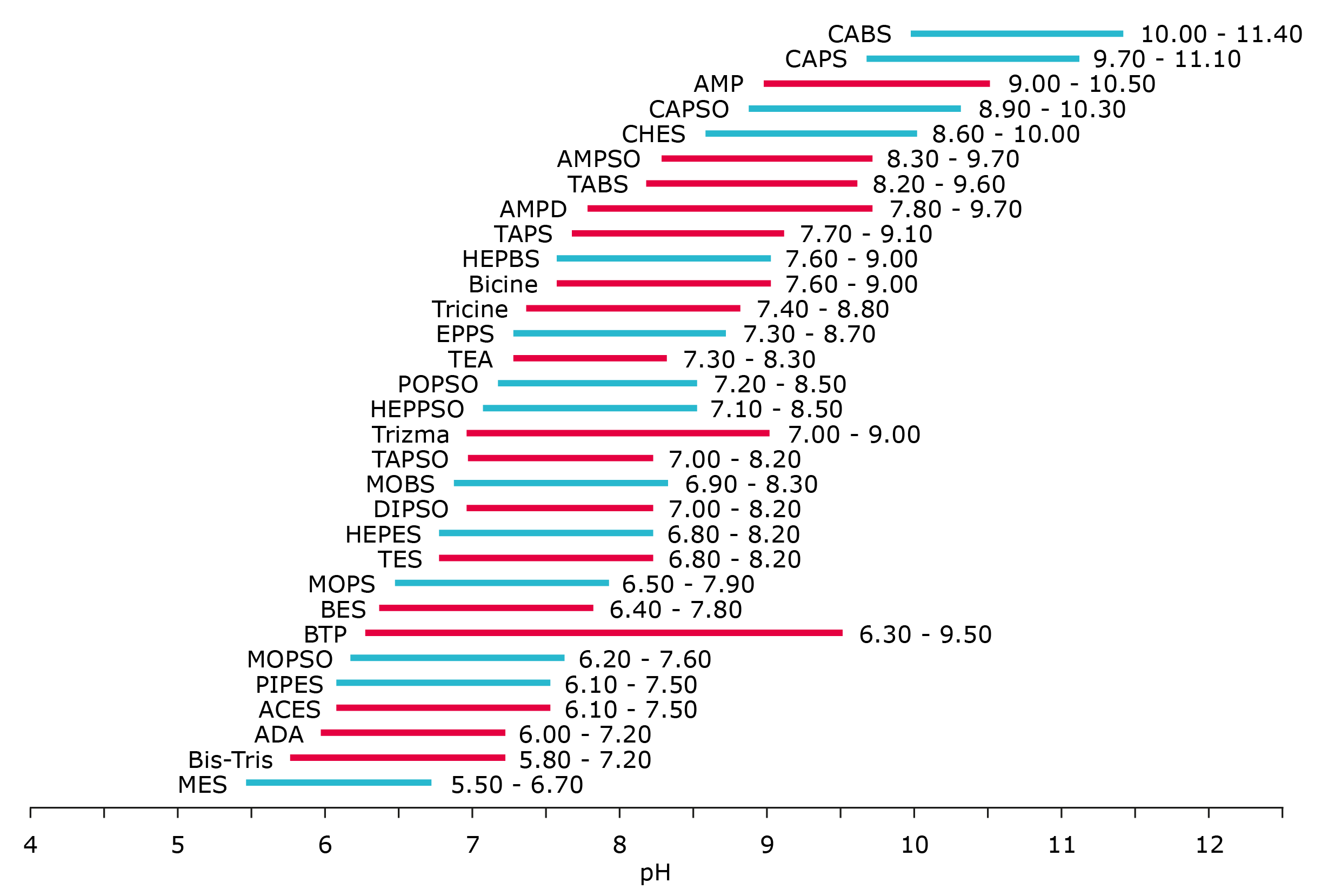
Figure 3. Interaction with Metals - Buffer suitability
- Cyan = Suitable for General Use
- Red = Not Suitable for General Use
This simplified chart shows the pH ranges for the various biological buffers. The cyan and red colors indicate whether or not the buffer is suitable for general use. The buffers in red should be used with caution when metals are employed or present in the biological system.
Such metal interactions can have an impact on:
- Cell growth and survival
- Interactions with cell membranes and macromolecules
- DNA, RNA, and protein measurement
- Redox studies
- Chromatographic separations
- Stabilization/destabilization of proteins
Thus, it is crucial to consider these properties while selecting an appropriate buffer for a particular experiment.
Improving Reproducibility with Biological Buffers
We have a broad range of biological buffers. The interactive buffer selection guide supports you in finding the right buffer according to pH, grade, packaging type, or application.
We are a primary manufacturer of high-quality biological buffers and has in-depth scientific knowledge, analytical capabilities and technical support for these essential products. With 25+ years of manufacturing experience, we are a strong, stable supplier with multiple, dedicated buffer manufacturing facilities to fulfill research and bulk orders (quantities ranging from a few grams to multi-metric tons). Moreover, we are ISO 9001:2008 certified and have controlled systems compliant with GMP or M-Clarity level to provide batch-to-batch consistency and supply chain transparency.
Practical Tips to Improve Reproducibility with Buffers
Knowing that there are potential concerns with the use of biological buffers, it is important to turn our attention to some of the practical tips that can be grouped into 3 main areas.

Figure 4. Improving Reproducibility with Buffers
1. Tips on Buffer Selectione):
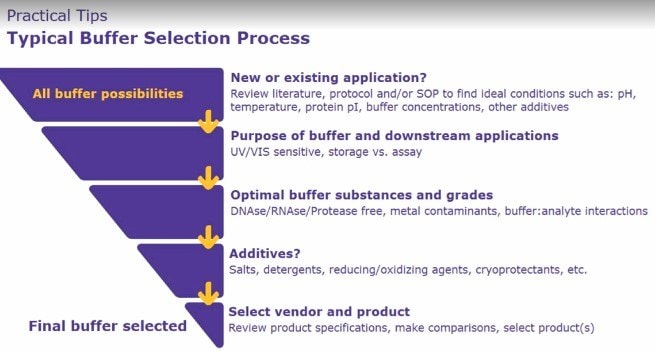
- The first reference for researchers to select the buffer should be previous literature, protocol and/or standard operating procedures in order to optimize the conditions, such as pH, temperature and so on.
- It is also a good idea to consider the purpose of the buffer and any downstream applications. For example, some buffer agents, such as ACES and ADA, can absorb light at different wavelengths that can affect downstream measurement.
- Selecting the optimal buffer substance to ensure that the buffer pKa is close to the desired pH to maximize the buffer capacity is crucial. Other concerns can also be addressed by selecting buffers that are free of any enzymatic activity, especially a product that is DNAse/RNAse or protease free. Also, if metals are a concern, select a buffer substance that has a low level of metal contaminants and does not chelate metals.
- Researchers often need to use additives, such as salts, detergents, etc., to improve protein stability or prevent oxidation-reduction or other aggregation. It is important to recognize possible buffer interactions with those types of additives.
- Lastly, selection of the vendor and the final buffer substance from the vast array of options that is available in the market, including the wide selection of buffer grades and quality, is essential. For example, low-end general use buffers may be selected for non-critical applications and more stringent products may be carefully chosen for pharmaceutical manufacturing. In addition, many different formats, such as powders, liquids, concentrates or ready-to-use products, should be wisely considered.
The table below illustrates the various buffer grades, according to the type of downstream application.
2. Tips on Buffer Preparation, Storage and Use:
These are general recommendations and depend on the application, industry, regulatory environment, and other factors.
- Buffers come in different commercially available formats, such as powder, ready-to-use products, liquid concentrates, etc. For solubilization or dilution of these products, high quality water (that is free from metal ions, organic matter, particulates and other contaminants) must be used by employing water purification systems, especially for critical applications. Other factors to consider in buffer preparation are the conductivity/pH of the water being used and working conditions, such as temperature and ionic strength as they affect the pKa. Since not all buffers can be autoclaved, submicron filtration may be employed to prevent contamination and growth.
- There are many references available to guide buffer storage, such as the guide on Good Laboratory Practice (GLP) and the electronic code of federal regulations in the US. It is a good practice to label buffers appropriately (indicating identity, titer or concentration, storage requirements and expiration dates) to ensure that they do not get switched or mistakenly used in laboratory or storage settings. Buffers that have expired or have become cloudy or discolored should be avoided as they are most likely to be contaminated. The storage conditions recommended by the manufacturers should be followed for vendor-supplied buffer solutions.
- Ideally, buffers should be prepared directly before use. If this is not possible, the previously recommended storage guidelines should be followed. It is always good to filter working solutions before use to ensure that they are free from any contamination or particulate matter.
3. Tips on Publishing Considerations
- Many publications lack important information about the key reagents used, including buffers. Many papers do provide the supplier and brand information of the buffers but may not report on a variety of information, such as grade or purity. Recommendations for documenting and reporting on buffers include buffer brand, product number, grade or quality, and batch or lot number. In addition, buffer composition, working concentration and reactions conditions, such as pH of the concentrate and the working solution, temperature at which the pH was measured, as well as the manufacturer and model of the pH meter contribute to making research more reproducible. Many publishers now realize the importance of providing such information and are making supplementary data repositories available for reference.
We have researched and developed a useful reference guide, which is an interactive buffer table with information about the useful pH ranges for each buffer, the working concentrations of the buffer, adverse metal ion interactions, pKa changes with temperatures, a variety of products or grades offered for that substance and also the intended applications.
Our aim is to solve the toughest problems in Life Science by collaborating with the global scientific community. By addressing and spreading awareness on research reproducibility issues, we hope that we can help researchers to overcome their hurdles in scientific investigations.
Our BioPerformance Certified buffers offer convenience, quality and reproducibility.
References
To continue reading please sign in or create an account.
Don't Have An Account?