Gel Electrophoresis
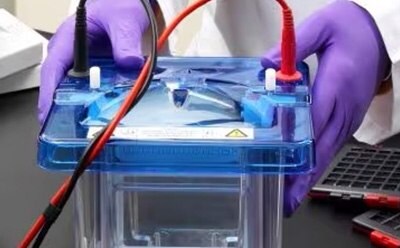
Protein gel electrophoresis is a common technique used to separate proteins for purification, characterization, and expression analysis. In this approach, charged protein molecules are transported through a gel by an electrical field. Their mobility through the electric field is dependent on protein size, shape, and charge.
Both polyacrylamide and agarose gel matrices can be used in protein electrophoresis. These matrices serve as a sieve, allowing smaller proteins to travel more rapidly than larger proteins. Agarose has a large pore size and can be used to separate proteins with radius larger than 5-10 nm, such as large protein complexes. Polyacrylamide has a smaller pore size, can separate proteins ranging in size from 5 kDa to 2,000 kDa, and is most commonly used in protein electrophoresis.
Featured Categories
Pre-stained and unstained molecular weight markers for protein gel electrophoresis, SDS-PAGE, and Western blotting applications.
Precast polyacrylamide protein gels, running buffers, and hand-casting reagents for hand-pouring polyacrylamide gels for PAGE and SDS-PAGE protein gel electrophoresis.
High sensitivity colorimetric and fluorescent protein gel stains, fixing solutions, and de-staining reagents for polyacrylamide gels, agarose gels, and PVDF and nitrocellulose membranes.
Electrophoresis tanks, blotting systems, and power supplies for protein gel electrophoresis and wet and semi-dry transfer.
Several methods of protein gel electrophoresis exist, each method providing distinct information for proteins of interest:
SDS-PAGE
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) enables protein separation based on molecular mass. In this method, SDS detergent is incorporated into the running buffer. The SDS imparts a net negative charge on proteins, masking their intrinsic charge. As proteins are separated in the presence of SDS and denaturing reagents, they become less globular and more linear. As a result, the rate at which SDS-bound proteins migrate through the gel is primarily dependent on their size, enabling estimation of molecule weight by comparing to protein standards.
Native PAGE
In native polyacrylamide gel electophoresis, proteins are separated in a way that preserves their native conformation (tertiary structure), subunit interactions (quaternary structure and protein-protein interactions), and biological activity. In this method, proteins are prepared and run under non-reducing, non-denaturing conditions. Protein mobility is determined by a complex combination of factors, as each protein can migrate towards either electrode depending on its charge, and at a rate dependent on its shape and binding behavior. For this reason, native PAGE is not recommended for molecular weight determination. Native PAGE is typically used in applications that require purification of active protein, or detection by an antibody that only recognizes the native form of the protein.
Isoelectric Focusing (IEF)
Isoelectric focusing uses both an electrical field and a pH gradient to separate proteins by their native isoelectric point (pI). As proteins move through the pH gradient, their net charge changes. Under an electric field, each protein migrates to the pH where its net charge is zero (termed as the isoelectric point of the protein). During the separation process, proteins in the sample accumulate, or "focus", in specific and predictable locations in the gel. Isoelectric focusing is used in protein identification from complex samples (e.g., cell and tissue lysates, plasma), analysis of post-translational modifications, and separation of samples for mass spectrometry analysis.
2D PAGE
Two-dimensional polyacrylamide gel electrophoresis enables resolution of protein according to both its intrinsic isolectric point (pI) and mass. Separation by pI occurs through isoelectirc focusing (IEF). Separation by mass occurs via SDS-PAGE. 2-D PAGE provides the greatest resolution for protein analysis, and is commonly used in proteomic research to resolve hundreds to thousands of proteins on a single gel.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Bis-Tris gels and buffers for superior protein resolution compared to traditional tris-glycine gels.
- DNA / Protein Electrophoresis and Troubleshooting Tables
- Identify causes and remedies for SDS-PAGE sample preparation challenges and optimize electrophoresis conditions.
- The Auto2D® 2-D Electrophoresis Device fully automates difficult 2D electrophoresis methods in a quick, easy, and reproducible way. Locate difficult-to-find proteins using the Auto2D® system in less than two hours with high reproducibility.
- Biological buffers are organic substances that maintain a constant pH over a given range by neutralizing the effects of hydrogen ions.
- See All (16)
Related Protocols
- Introduction to PAGE. Learn about SDS-PAGE background and protocol for the separation of proteins based on size in a poly-acrylamide gel.
- TAE and TBE are both used as running buffers for nucleic acid electrophoresis but have some important differences. Review our recipes and video to give your application the best chance of success.
- Learn Northern and Southern blotting basics, with protocols and applications for macromolecule transfer to membrane supports.
- Brilliant Blue G-Colloidal Concentrate stains proteins in IEF, PAGE, and SDS-PAGE gels after electrophoresis, enhancing sensitivity with protein fixation.
- This protocol shows how to insert gels into Ettan™ DALT electrophoresis units.
- See All (21)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?



