细胞库制造
您的主细胞库(MCB)对治疗性产品开发至关重要,同时还能支持生物制品的临床开发、生产及商业供应。让我们在美英两国的cGMP设施中为您处理MCB生产,并同步制备工作细胞库(WCB),确保首次即达标——这将保障高质量并为后续生产提供便捷的规模化路径。
我们可在传统或封闭工艺设施中生产高达1,000瓶的细胞库。凭借行业领先的生产经验,我们可处理多种细胞系:
|
|
|
封闭式细胞库生产
封闭式处理专为您的悬浮细胞系设计。我们的团队在完全封闭的系统中培养您的细胞,并采用详尽且有据可查的污染控制策略进行评估。选择封闭工艺生产细胞库可降低交叉污染风险,同时实现自动化操作,从而减少人为失误。
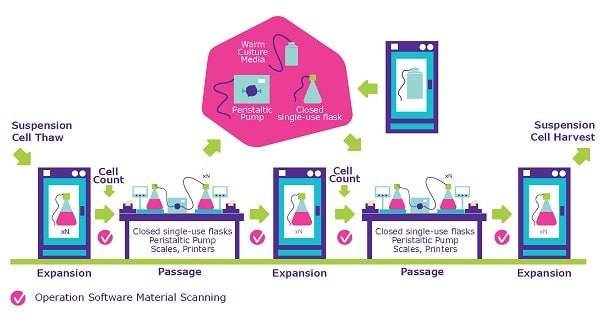
封闭式加工通过封闭膨胀过程中的各个步骤,最大限度地降低了污染物的可能性。
相关资源
- Infographic: Cell Line Preparation for Optimal Production
In this infographic, we show the 3 main steps for optimal cell line preparation that will give you peace of mind for your production process.
- Technical Note: Transition to Closed Processing Systems
Read this tech note to see how commonly banked cell types fared using traditional versus closed culture systems.
- Flyer: Confidence, Closed Processing Cell Bank Manufacturing
Discover how closed processing reduce the risk of cross-contamination in your cell bank manufacturing.
- Flyer: Cell and Virus Bank Development, Characterization, and Storage You Can Count On
We ensure your cell lines are produced and maintained for optimal production.
索取信息
相关网络研讨会
观看本次网络研讨会,了解如何充分利用您的细胞库。
在本场网络研讨会中,我们将通过案例研究和实际操作示例,带您逐步了解GMP生产流程。
如要继续阅读,请登录或创建帐户。
暂无帐户?
