Optimizing Upstream Processing: Media Filtration and Cell Culture Performance
Dry powder cell culture media is typically reconstituted at the point of use and then sterilized with a high temperature for a short period of time (HTST) or filtered through a sterilizing-grade membrane filter before being transferred into the bioreactor.
When selecting a membrane filter for processing cell culture media, microbial retention objectives should be considered, along with membrane area requirements and planned process conditions. Before use, it is highly recommended to confirm the filtered media supports the required cell culture performance to meet process objectives. Confirmation of performance should guide filter selection.
This page provides guidance on selecting and sizing filters for cell culture media filtration. Acceptable performance of the filtered media was then confirmed with a CHOZN® GS antibody-producing CHO cell line and EX-CELL® Advanced CHO fed-batch medium.
Selecting and Sizing Filters for Cell Culture Media
Selecting a membrane filter for processing cell culture media is influenced by the level of microbial retention, compatibility of the filter materials with the fluid stream, and the filter capacity for the specific media.
Microorganism Retention
Risk assessments of materials, processes, and the manufacturing environment guide the level of microorganism retention required from the cell culture media filter. While traditional sterilizing-grade filters are rated to have a membrane pore size of 0.2 μm, some adventitious agents such as mycoplasma, can penetrate these filters, resulting in bioreactor contamination. Mycoplasma-retentive (0.1 μm) filters are an alternative to sterilizing-grade filters and minimize the risk of contamination from smaller adventitious agents. However, while these smaller pore filters reduce the risk of bioreactor contamination, they may result in a lower flux and lower filter capacity.
Filter Capacity
Filter capacity depends on the structure of the filter membrane and the cell culture media formulation, which can vary widely. Proper hydration of powdered media is essential for achieving consistent, scalable, filter capacity as batch-to-batch variability can impact filter performance. As a result, filtration area sizing calculations typically incorporate safety factors to account for fluctuations in throughput or filter capacity.
While smaller pore size filters reduce the risk of bioreactor contamination, they may also lower filtration flux and capacity. Membrane composition, structure, surface chemistry, and layering all influence permeability and capacity, leading to differences in filtration area requirements, footprint, and cost of goods. Equally critical is the potential impact of filtration on cell culture performance as the filtration solution must support achieving the cell culture performance objectives in terms of viable cell density (VCD) and productivity.
The cell culture media and polyethersulfone (PES) filters used in these studies are listed in Tables 1 and 2, respectively. Approximately 500 mL of each medium was processed through each filter. Filtration area requirements for the various filters were determined using the Vmax™ method, which estimates the minimum filtration area (Amin) required to meet process requirements.
Figure 1 illustrates a typical Vmax™ test setup.1 Volumetric throughput data was fitted to filter pore plugging models to determine the minimum filtration area required for each filter-medium combination.2,3 These values were then used to recommend appropriate filter capsule sizes, incorporating a safety factor to ensure reliable performance.
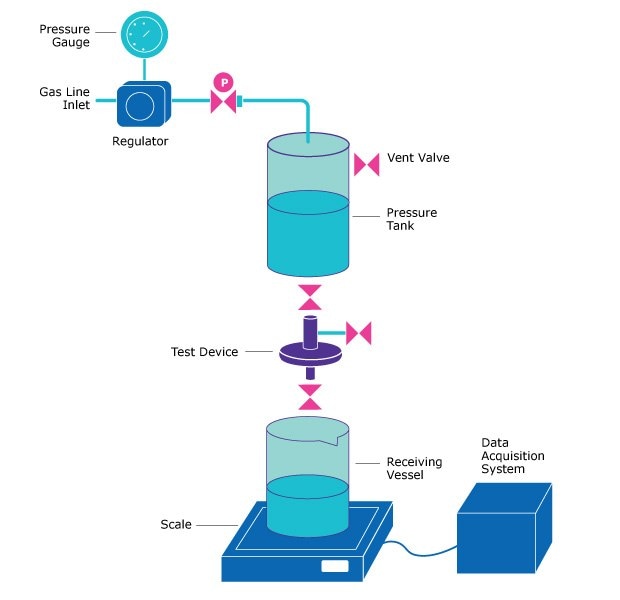
Figure 1.Setup for Vmax™ constant pressure filtration studies.
Filter Throughput Results
Figure 3 shows the throughput for the various cell culture media processed through the different filters. As cell culture media passed through the filters, the membrane pores became restricted, reducing flow, leading to reduced flux and lower throughput over time.
The tested filters also exhibited differences in permeability due to differences in membrane structure, surface chemistry, and presence or absence of an onboard prefilter. Millipore Express® SHC and Millipore Express® SHRP filters contain an onboard 0.5 μm PES prefilter, which removes larger particles and protects the smaller pore size sterilizing-grade membrane. For cell culture media with pugging components, the presence of the onboard prefilter can enable higher throughput and increase filter capacity.
Results indicated that Millipore Express® SHC filters achieved the highest throughput of all sterilizing-grade filters for all cell culture media and feeds tested. For filters designed to reduce mycoplasma and remove bacteria, Millipore Express® SHRP filters achieved the highest filtration throughput with most of the cell culture media. For biopharmaceutical manufacturers with low tolerance for bioreactor contamination, these filters offer an effective risk mitigation solution.
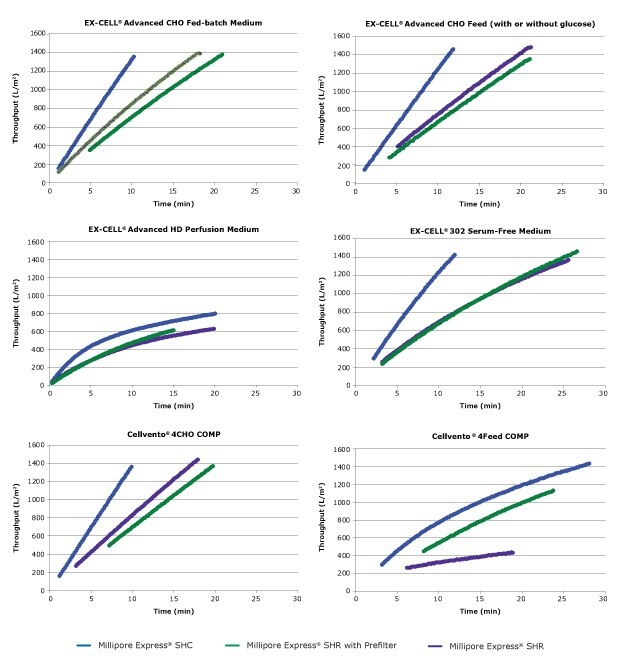
Figure 2.Throughput of catalog media on filters with different microbial retention characteristics; the type of media is shown in the title of each graph.
Throughput data were processed using the models to estimate the filtration area. Sample results for one medium/feed combination are shown in Table 3; note that a safety factor was not included in Amin calculations. For this non-plugging medium, the prefilter in Millipore Express® SHRP did not improve throughput, and the additional resistance of the prefilter layer may have increased filtration area requirements.
Recommended filters for processing different volumes of various cell culture media are provided in Table 4. The sizing recommendations include a minimum safety factor of 1.5.4 For non-plugging media, the size of the filter outlet should be considered to ensure flow is not affected.
Evaluation of Cell Culture Media Performance
Prior to implementing a filter for processing cell culture media, a study to assess cell growth in the filtered media should be performed. Such studies complement Vmax™ studies and are an essential element of filter selection.
Cell Growth Study in Spin Tube Bioreactors
Spin tube bioreactors were inoculated with CHOZN® GS antibody-producing CHO cells at 0.3 x 106 cells/mL in 25 mL filtered cell culture medium. Table 5 lists the different filters used to process the culture media. The 0.22 µm Durapore® filter is made of polyvinylidene fluoride (PVDF) and served as a benchmark for cell culture media performance following filtration.
Spin tubes were cultured in an incubator at 37.0 °C, 80 % relative humidity, 5.0 % CO2, 300 RPM agitation speed. Samples were collected daily from each bioreactor and analyzed for VCD and viability. Sampling was stopped when peak VCD was reached.
Cell Growth Study Results
The following study evaluated cell viability and cell growth of CHOZN® GS cells in EX-CELL® Advanced CHO Fed-batch medium filtered with Millipore Express® membranes. Cell growth assessments were performed using spin tube bioreactors. To detect any potential negative impact of leachables or adsorbed components from the filter or filtration process on cell growth more easily, the ratio of membrane area to medium volume was maximized. This test condition represents a relatively low filtration throughput, corresponding to ‘worst-case’ conditions. The results of viable cell density and viability profiles are shown in Figure 3.
The VCD profiles for all test conditions were similar; differences between the ‘worst-case’ and ‘low throughput’ filtration conditions were minimal. Moreover, cell density profiles were consistent regardless of which membrane filter was used. These results confirm that the membrane filter did not impact growth of the cells in EX-CELL® Advanced CHO Fed-batch medium.
Similarly, there were minimal differences in cell viability between the tests with different membrane filters or filtration volumes. Cell viability remained high throughout the culture for all test conditions. These results demonstrate no negative interactions between the filter and cell culture medium that might impact cell viability.
The data set demonstrated that filtering cell culture media through either 0.1 μm or 0.2 μm Millipore Express® PES membrane filters yielded comparable cell culture performance to that achieved with traditional PVDF membrane filters.
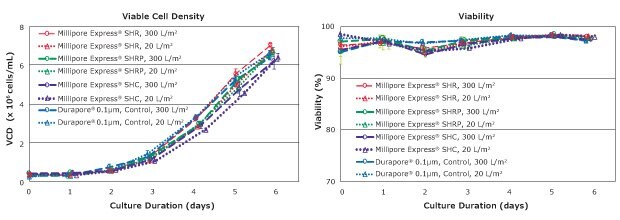
Figure 3.Viable cell density and viability of CHOZN® GS cells in filtered EX-CELL® Advanced CHO Fed-batch Medium. Each data point represents the average of triplicate runs, with error bars representing one standard deviation.
Conclusion
Selecting the appropriate filter for cell culture medium and feeds is a critical step in ensuring process efficiency and maintaining cell viability in upstream processes. By incorporating risk assessment, throughput testing to calculate filtration area, and cell culture studies to confirm acceptable cell culture performance, informed decisions that optimize filtration performance can be made.
These studies demonstrated that filtration through PES membrane filters with pore sizes of 0.1 μm or 0.2 μm did not negatively impact CHOZN® GS cell viability or density, even under worst-case low-throughput conditions and serve as a useful benchmark for filter selection. Due to variability in cell lines, media formulations, and processing conditions, it is recommended that filtration studies be conducted within the specific parameters of each bioprocess.
A comprehensive evaluation, including additional process components such as single-use consumables, mixing systems, and fluid handling, can further ensure the robustness of filtration strategies. By taking a systematic approach to filter selection and validation, upstream process developers can enhance the reliability and scalability of their cell culture operations.
For help determining the right filter for your process, contact us.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?