Sample Matrix Problem Resolution
In the flavor and fragrance industry, analytical chemists are often required to isolate key ingredients, off-flavor components, or trace organic compounds from their sample matrices without altering their structure or composition. Traditional approaches to isolating the analytes of interest include solvent extraction, purge and trap analysis and static headspace analysis. Solid Phase MicroExtraction (SPME) offers an approach that provides the benefits of these methods while at the same time eliminates the use of solvents and reduces analysis costs and the time required for sample preparation. Essentially all sample matrices, whether liquid, solid or gaseous, can be sampled by SPME.
SPME is a solvent-less extraction procedure that involves the exposure of a coated fused silica fiber to a gaseous or liquid sample or the headspace above a liquid or solid sample. The fiber coating is typically an immobilized polymer, a solid adsorbent or a combination of the two. Published articles, many of which are referenced in our SPME application guide, have sited the use of SPME for sampling complex matrices or matrices containing interfering organic compounds. Past issues of the Reporter Newsletter have demonstrated the success of SPME in resolving difficult matrix problems such as the isolation of pyrazines from peanut butter, fatty acids from milk products, and amphetamines from biological fluids. By sampling the headspace above a complex sample matrix, SPME helps to minimize effects due to interfering organic compounds. Also, headspace-SPME can provide a representation of the organic composition of the sample for screening applications or can isolate and concentrate specific trace analytes of interest for quantitative applications. Through the evaluation and adjustment of sample conditions such as temperature, pH and fiber exposure time, SPME extraction efficiency and sensitivity can be optimized. Likewise, sample agitation and analyte solubility reduction through salting effects are common practices that improve the extraction efficiency.
Extraction of Flavor Components from Orange Juice
In a recent publication by Dr. Rouseff from the University of Florida, SPME was used for the identification of volatile flavor components in an orange juice matrix.1 Dr. Rouseff compared the results of SPME extraction to gas chromatography-olfactometry (GCO), a technique that utilizes trained odor panelists for the identification of volatile flavor components. SPME was demonstrated to be able to detect concentrations below FID response levels, yet verifiable by GCO at a sensor port. Since SPME does not utilize solvents, early eluting volatile components are not masked by a solvent peak, a common problem encountered when using other extraction techniques. The chromatogram in Figure 1 shows a typical orange juice extract obtained using SPME and the Carboxen/PDMS fiber. Headspace sampling was conducted over the orange juice sample for 15 minutes. This allowed the extraction of the volatile compounds such as acetaldehyde as well as the lower volatility terpenes and sesquiterpenes. By increasing the exposure time of the fiber, extraction of the higher boiling compounds is enhanced at the sacrifice of the more volatile aldehydes. The dual coated DVB/Carboxen/PDMS fiber is under investigation to determine if it offers the advantage of extracting a broader range of analytes from the citrus sample matrix.
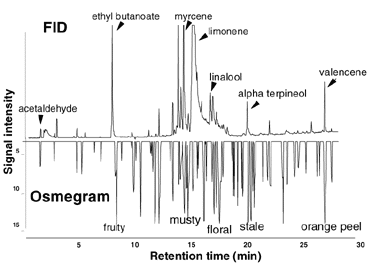
Figure 1. Flavor Components in an Orange Juice Matrix
Conditions
Sample:
25 mL orange juice
Fiber:
75 µM Carboxen-PDMS
Extraction :
headspace, 30 min. @ 40 °C stirred
Desorption:
3 min., 320 °C
Column:
30 m, 0.25 mm I.D., 5% methyl-phenyl siloxane
Oven:
3 min. @ 32 °C, 6 °C/min to 200 °C
Carrier:
Helium @ 29 cm/sec.
Injection;
320 °C, 0.75 mm I.D. inlet liner splitless
Detector:
FID
Extraction of Sulfur Compounds from Saliva
Another complex matrix problem handled by SPME was the extraction of volatile sulfur compounds from saliva and breath (oral malodors). This was demonstrated by Richard Payne of the Colgate Palmolive Company at the ACS National meeting in August, 1999 (2). Payne faced significant challenges in dealing with the complex nature of the oral cavity. Not only does the sample matrix change with time, but there were also difficulties to overcome in physically sampling and concentrating the sulfur malodors. Extraction techniques such as off- line closed-loop trapping, purge and trap, and direct injection of mouth air samples have been tried in the past. The approach taken by Payne was to collect saliva samples from human subjects, incubate the saliva overnight at 37 °C, and conduct dynamic headspace sampling over the sample. The Carboxen/PDMS fiber was positioned in a specially designed sampling tube that allowed the fiber to collect the malodors from a stream of air passing over the saliva sample. Sample collection was performed for one minute prior to GC-MS analysis. Figure 2 shows the results of the extraction. The ability of the Carboxen/PDMS fiber to collect a variety of sulfur-containing compounds was demonstrated. Hydrogen sulfide, considered to be one of the prime components of oral malodors, did not show good extraction efficiency with the Carboxen/PDMS fiber. However, many new compounds were identified from the SPME headspace sampling approach and are indicated in Figure 2 with a (*) symbol.
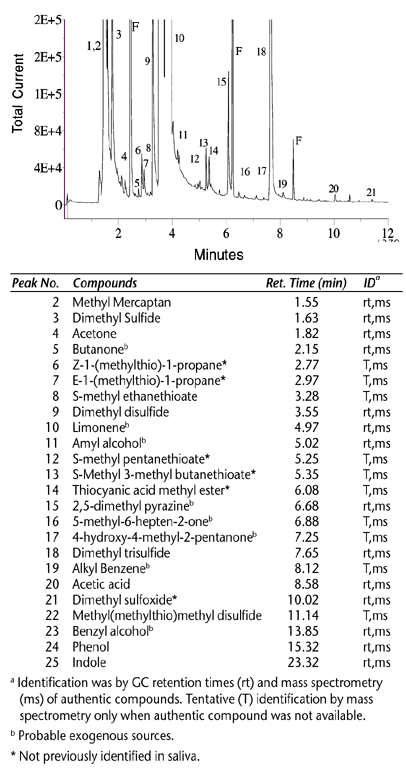
Figure 2. Sulfur Components in a Saliva Matrix
Conditions
Sample:
3 mL saliva solution w/thioglycollate medium
Fiber:
75 µM Carboxen-PDMS
Extraction :
headspace, 15 min. @ 22 °C, stirred
Desorption:
1 min., 250 °C
Column:
30 m, 0.25 mm I.D., 0.25 µM, Supelcowax 10
Oven:
50-200 °C, 10 °C/min hold 5 min. @ 200 °C
Carrier:
Helium @ 30 cm/sec.
Injection:
250 °C, 0.75 mm I.D. inlet liner, splitless
Detector:
GC-MS ion trap
In both examples above, SPME was used to overcome the complexity of the sample matrix by sampling the headspace above the sample. Eliminating the interfering matrix components improves the ability of the analyst to identify key compounds that may otherwise be masked. SPME fibers coated with Carboxen/PDMS have been demonstrated as the best fiber choice for the collection of volatile organics.
Analysts in the food and beverage industry continue to use SPME for applications involving the extraction of flavors and odors (3); examples demonstrated in this reference include analysis of off-flavors in milk (arising from exposure to light), caffeine in coffee, aroma compounds in apples, analysis of rancid potato chips, volatiles in candy, and more. If your needs are to identify volatile organic compounds in complex sample matrices, consider the advantages of SPME, Sample Preparation Made Easy.
Acknowledgement
Information and illustrations in this article were submitted by:
Dr. Russell Rouseff, Univ. of Florida,
Citrus Research and Education Center,
Lake Alfred, FL
Mr. Richard Payne, Colgate Palmolive
Co., Piscataway, NJ
References
如要继续阅读,请登录或创建帐户。
暂无帐户?