Olivine-Type Cathode Materials for Li-Ion Batteries
Would you like to be notified by email when we publish new technical papers related to battery development? If so, click the subscribe button to complete a short form. Your information will be kept private, and you can unsubscribe at any time.
Introduction
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance. Currently, LIBs are used in electric vehicles and hybrid electric vehicles as well. In these batteries, olivine-type cathode materials such as LiMPO4 (M=Fe and Mn) have attracted significant interest, especially due to their low cost and high intrinsic safety. However, they show poor electrochemical properties mainly due to their low electrical conductivities. Thus far, a number of attempts have been made to enhance the electrochemical properties of olivine-type cathode materials, including particle-size reduction,1 cation doping,2 carbon coating of LiMnPO4,3 and use of LiMnPO4/carbon composite.2,4 Several synthesis routes have been developed to overcome the weakness of LiMnPO4 3 such as solid-state reaction,4–6 sol-gel method,2,3 polyol process,7 hydrothermal synthesis8 precipitation,9 and electrostatic spray deposition.10 However, the electrochemical performance of LiMPO4 cathodes prepared using the aforementioned techniques does not yet meet the application needs of commercial cells.
Spray pyrolysis (SP) is a well-known continuous and single-step method for the preparation of fine homogeneous high-purity multicomponent powders. Compared to particles prepared by soft chemistry methods, SP yields a narrow particle size distribution that can be controlled from the micrometer to sub-micrometer scale. Moreover, SP can be used to achieve highly pure powders with easily controllable composition. About a decade ago we first reported that the spherical nanostructured LiMn2O4 and its substituted forms could be prepared by spray pyrolysis.11,12 Recently, we have also reported that LiMPO4/C (M=Fe, Mn, Co) nanocomposites13–16 with excellent electrochemical properties can be successfully synthesized using a combination of SP, ball milling, and heat treatment. Some of our recent results on LiMPO4/C composites are summarized in this article.
Synthesis and Electrochemical Properties of LiFePO4/C Nanocomposites
LiFePO4/C nanocomposites were prepared at 500 °C by SP and then wetball milled (WBM) at a rotating speed of 800 rpm. The WBM with ethanol was performed for 3 h in an Ar atmosphere, and the milled mixture was heat-treated at 600 °C for 4 h in a N2+3% H2 atmosphere. Transmission electron microscopy (TEM) revealed that the LiFePO4 nanoparticles, with a geometric mean diameter of 146 nm, were coated with a thin carbon layer of several nanometers (Figure 1).
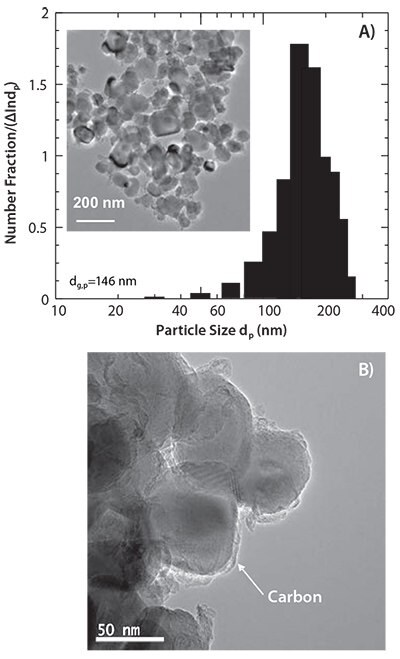
Figure 1.TEM images and particle size distribution of the sample prepared by the combination of spray pyrolysis and wet-ball milling followed by heat treatment.
The first charge-discharge profiles of cells containing carbon-coated LiFePO4 nanoparticles with increasing charge-discharge rate from 0.1 to 60 C between 2.5 and 4.3 V are presented in Figure 2. At a charge-discharge rate of 0.1 C, the cell has a discharge capacity of 165 mAh g-1, which corresponds to 96% of the theoretical capacity of LiFePO4 (170 mAh g-1) and a much smaller polarization loss and irreversible capacity. A wide flat voltage plateau corresponding to the Fe+2/Fe+3 redox reaction is observed at 3.4 V. The first discharge capacities at charge-discharge rates of 1, 5, 10, and 20 C are 155, 130, 118, and 105 mAhg-1, respectively. Even at a rate of 60 C, the electrode containing carbon-coated LiFePO4 nanoparticles has a discharge capacity of 75 mAh g-1.
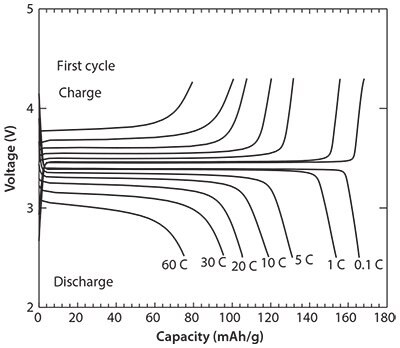
Figure 2.Charge-discharge curves at 1st cycle of carbon-coated LiFePO4 nanoparticles.
The cyclic performance of the cells containing carbon-coated LiFePO4 nanoparticles at different charge-discharge rates was investigated over 100 cycles, and the results are given in Figure 3. The cells exhibit an excellent cyclic property. The cells show no capacity fading even after 100 cycles at different charge-discharge rates ranging from 1 to 60 C. These results demonstrate that the structure of the carbon-coated LiFePO4 nanoparticles is very stable, and the electrochemical lithium-ion insertion/extraction process is quite reversible even at high chargedischarge rates. These results indicate that the preparation approach using the combination of SP and WBE followed by heat treatment enables to achieve a high rate performance for LiFePO4 composite electrode materials.
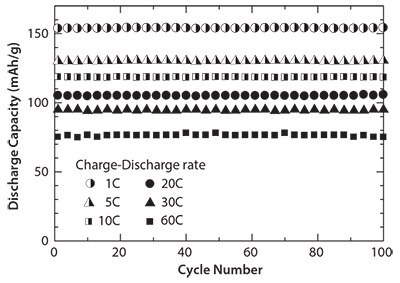
Figure 3.Cycle performance of carbon-coated LiFePO4 nanoparticles.
Synthesis and Electrochemical Properties of LiMnPO4/C Nanocomposites
LiMnPO4/C nanocomposites were prepared using a combination of SP, WBM and heat treatment. The SP was performed at 300 °C, whereas the heat treatment was performed at 500 °C for 4 h in a N2+3% H2 atmosphere. X-ray diffraction (XRD) was used to confirm the ordered LiMnPO4 olivine structure without any impurity phases. The scanning electron microscopy (SEM) and TEM also confirmed that the final sample was the LiMnPO4/C nanocomposite with a primary particle size of 100 nm. The TEM of the final sample is shown in Figure 4. Both phases of carbon and LiMnPO4 could be seen in this image.
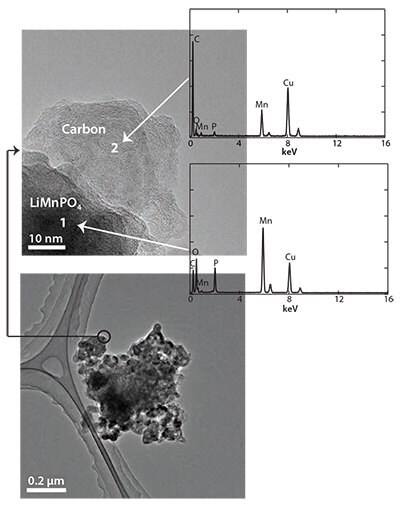
Figure 4.TEM images of the LiMnPO4/C nanocomposite prepared by a combination of SP (at 300 °C) and WBM followed by heat treatment at 500 °C.
Figure 5 shows the initial charge-discharge profiles of the cells evaluated at constant current (CC)-constant voltage (CV) charge mode up to the cutoff voltage of 4.4 V and then CC discharge at various rates. The flat discharge plateau could still be recognized even at discharge rate of 2 C. This is attributed to the deep Li extraction level under very low currents in CV charge stage.
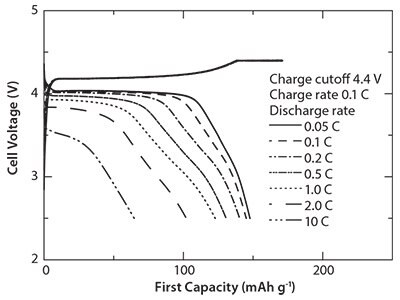
Figure 5.First charge/discharge curves at CC-CV charge condition of the cells containing the LiMnPO4/C nanocomposites prepared by the combination of SP (at 300 °C) and WBM followed by heat treatment at 500 °C.
Figure 6 shows the rate capabilities determined for three charging modes: (1) CC charge up to 4.4 V, (2) CC charge up to 4.8 V, and (3) CC charge up to 4.4 V followed by CV charge reaching to theoretical capacity for LiMnPO4/C nanocomposite containing cell. At the discharge rate of 0.05 C, the cell exhibits the first discharge capacity of 123, 153, and 147 mAh g-1 in CC-4.4 V, CC-4.8 V and CC-CV modes, respectively. At a discharge rate of 1 C, the initial discharge capacity of 51, 107, and 123 mAh g-1 were observed for CC-4.4 V, CC-4.8 V and CC-CV modes, respectively. Even with a discharge rate of 10 C, the cell could deliver capacity of 65 mAh g-1 in CC-CV mode. In spite of the intrinsic low electronic and ionic conductivities of LiMnPO4, the cells containing LiMnPO4/C nanocomposites exhibit very good rate capability, which could be attributed to the large specific surface area, the small primary particle size, and a uniform carbon distribution.
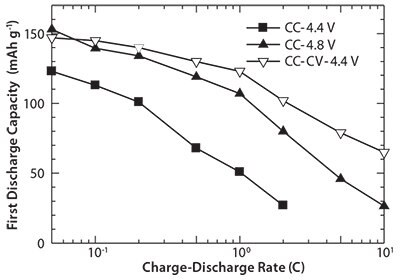
Figure 6.Rate capability of the cells containing the LiMnPO4/C nanocomposites prepared by the combination of SP (at 300 °C) and WBM followed by heat treatment.
The cycle performance comparison between three charge modes is shown in Figure 7. The cells containing LiMnPO4/C nanocomposites show good cycle performance for all the three different charging modes.
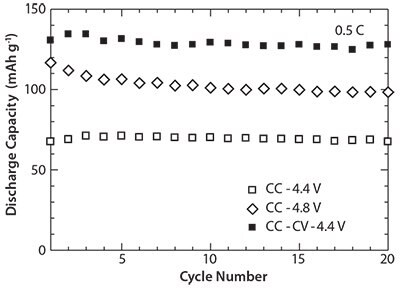
Figure 7.Cycle performance at different charge modes of the cells containing the LiMnPO4/C nanocomposites prepared by the combination of SP at 300 °C and WBM followed by heat treatment.
Synthesis and Electrochemical Properties of LiCoPO4/C Nanocomposites
LiCoPO4/C nanocomposite also was successfully prepared by using a combination of SP and WBM followed by heat treatment. The SP was performed at 300 °C, whereas the heat treatment was performed at 500 °C for 4 h in a N2+3% H2 atmosphere. The XRD analysis confirms that the LiCoPO4/C nanocomposite is well crystallized in an orthorhombic structure with Pmna space group. SEM and TEM equipped with energy dispersive spectroscopy technique reveals that the LiCoPO4/C nanocomposites are agglomerates of LiCoPO4 primary particles with a geometric mean diameter of 87 nm, and the carbon is well distributed on the surface of the agglomerates.
Figure 8 presents the first discharge profile of the cells containing LiCoPO4/C nanocomposites as active cathode materials at 0.1 C. For comparison, the discharge profile of cells containing LiCoPO4 is also shown in the same figure. The LiCoPO4/C nanocomposite cathode exhibits a wide and flat voltage plateau around 4.75 V. It can be noted that in comparison to LiCoPO4, the LiCoPO4/C nanocomposite cathode delivers a larger discharge capacity (94 mAh g-1 for LiCoPO4 vs. 141 mAh g-1 LiCoPO4/C nanocomposite). Figure 9 shows the rate capabilities of the cells containing LiCoPO4 and LiCoPO4/C nanocomposites. The cells containing LiCoPO4 exhibit first discharge capacities of 99, 94, 78, 53, and 11 mAh g-1 at 0.05, 0.1, 1, 5, and 20 C, respectively; the cell containing LiCoPO4/C nanocomposites shows first discharge capacities of 142, 141, 137, 128, and 109 mAh g-1 at 0.05. 0.1, 1, 5, and 20 C, respectively.
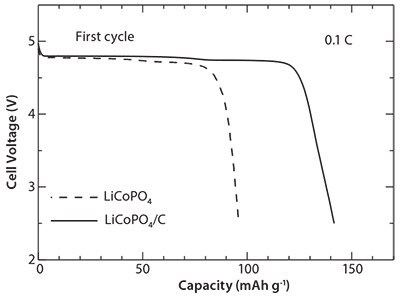
Figure 8.First discharge profiles of the cells containing LiCoPO4/C nanocomposite cathode at 0.1 C.
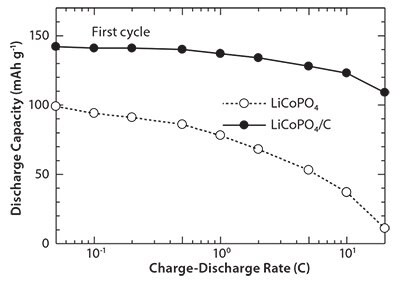
Figure 9.Rate capabilities of the cells containing LiCoPO4/C nanocomposite cathode.
Conclusion
To summarize, the LiMPO4/C (M=Fe, Mn, Co) nanocomposites were successfully synthesized using a combination of spray pyrolysis, ball milling, and heat treatment. The LiMPO4/C nanocomposites synthesized using this approach show excellent electrochemical properties with the specific capacities close to that of theoretical values. The results indicate that the combination of spray pyrolysis and ball milling aerosol has the potential to be an effective synthesis route for improving the electrochemical properties of olivine-type cathode materials for lithiumion batteries.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?