Recent Developments in Magnetic Iron Oxide Nanoparticles for Non-Invasive Bioimaging
Introduction
Non-invasive imaging is a valuable tool for the detection and diagnosis of disease, as well as for monitoring patients’ response to treatment. With the rapid advances in technology and increased availability and accessibility of imaging equipment and capability, the number of radiological examinations has grown significantly in the past decade — an estimated 280 million clinical imaging tests are performed annually in the US alone.1,2 Disease-specific and targeted imaging and drug delivery techniques have been actively pursued in recent years for precision medicine, where biomarker targeting, high sensitivity, and high resolution are often required. Imaging contrast agents are often used to enhance both contrast and sensitivity of lesion detection and quantitative measurements of physiological and pathological conditions, thus enabling more accurate diagnoses and proper treatment decisions. Many new nanomaterial-based contrast agents and imaging probes have been introduced, promising to expand the capabilities and applications of non-invasive imaging, as well as image-guided interventions, to advance clinical practice. For example, magnetic resonance imaging (MRI) is one of the most informative diagnostic imaging modalities because it is radiationfree, and offers high spatial resolution, soft tissue contrast, and reveals diverse image features associated with various tissue characteristics. The unique chemical and physical properties of magnetic nanomaterials offer many advantages for non-invasive, biomarker-targeted MRI applications,3 especially when compared to clinically used, low molecular weight gadolinium (Gd) chelate contrast agents. These FDA approved Gd3+-chelates, e.g., Gd-DTPA (Magnevist®), can amplify the signal or contrast of the tissue under examination based on changes in longitudinal relaxation time, i.e., T1, because the seven unpaired electrons of paramagnetic Gd3+ have high longitudinal relaxivity (r1 ≈ 4–8 mM−1·s−1), which can be further increased by properly selecting the chelate ligands.
On the other hand, superparamagnetic iron oxide nanoparticles (SPIO or IONPs) typically provide a different contrast-enhancing mechanism. They shorten the transverse relaxation time, i.e., T2 and T2*, by generating imaging contrast that mainly suppresses the signal from affected tissue or organs. While clinical uses of magnetic IONP-based contrast agents, e.g., Feridex®, have declined significantly in recent years due to their limited applicability, IONPs have been widely used to develop platforms for targeted molecular imaging and theranostic applications that take advantage of the unique properties of magnetic nanomaterials. In addition to their excellent biocompatibility, IONPs have 1) strong and tunable magnetic properties, which result in superb contrast-enhancing effects, 2) favorable pharmacokinetic properties with prolonged blood and tissue retention times, and 3) diverse surface chemistry, which allows functionalization of IONPs for multimodal imaging, biomarker targeting, and delivery of therapeutics as illustrated in Figure 1. With recent advances in molecular imaging and the increasing need for precise, functional imaging for both diagnosis and therapy, there is renewed interest in developing new IONPs for use in biomedical imaging.
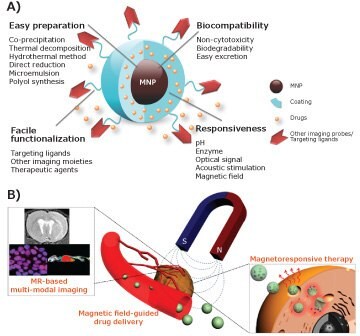
Figure 1. Magnetic nanoparticles (MNP): A) Advantages and properties for developing imaging applications, and B) some examples of biomedical applications. Adapted with permission from references 5 and 7 copyright 2016 Wiley-VCH and copyright 2015 ACS respectively.
Since many excellent reviews have recently been published describing the chemistry, properties, and imaging applications of MNP, including ours,4,5 this article will focus on three areas of magnetic IONPs: 1) shape control and renal-clearable sub-5 nm IONPs with T1 or dual T1-T2 MRI contrast enhancing capabilities, 2) magnetic particle imaging (MPI) applications that solely depend on specific IONPs, 3) multifunctional IONPs for use in hybrid MRI (MRI combined with optical and/or nuclear imaging).
Sub-5 nm IONPs as T1 or T1-T2 Dual MRI Contrast Agents
While IONP-based agents are highly biocompatible, and are in fact used for clinically treating iron deficiency, there are increasing concerns about poor clearance and long liver retention time for the large IONPs (i.e., 10–50 nm core) currently used in biomedical imaging applications. Moreover, increasing incidents of severe nephrogenic systemic fibrosis (NSF) caused by free Gd3+ ions leaching out of their complex, limit the clinical applications of Gd-based T1 contrast agents from patients with renal dysfunctions. This has accelerated efforts to develop IONPs with both excellent T1 contrast and a better clearance profile.
Traditionally, IONPs are used as T2 “darkening” contrast agents, because nearby magnetic IONPs affect the T2 relaxation time of the hydrogen atoms in water molecules, a main source of the signal for MRI. Transverse relaxivity (r2) correlates directly with the IONP size. As IONP size increases from 4 to 6, 9, and 12 nm, r2 values gradually rise from 78 to 106, 130, and 218 mM-1·s-1 (at a field strength of 1.5 T). The high r2 values and r2/r1 ratios (ca. 10) of conventional IONPs, with core size ranges from 10–50 nm, make IONPs excellent T2 contrast “darkening” agents, but not the clinically preferred T1 “brightening” contrast agents.5 In order to “tune” the relaxivities for gaining T1 contrast, low dimensional IONPs were developed, including ultrathin iron oxide nanowhiskers with diameters of less than 3 nm and ultra-fine iron oxide nanoparticles (uIONPs) were developed.6,7 Shown in Figure 2A–C, ultrathin iron oxide nanowhiskers that were 20 nm long and 2 nm in diameter with r1 of 6.13 mM-1·s-1 and r2/r1 ratio of 1.83 enhanced the T1 contrast in T1 weighted spin echo images of both phantoms and rats received subcutaneous and intraperitoneal injection of nanowhiskers.
Sub-5 nm uIONPs also show promise as T1 weighted contrast agents, due to their biocompatibility and their Gd3+-comparable r1 value (4–5 mM-1·s-1) and low r2/r1 ratio (<4) resulting from the ultra-small size (below 5 nm) and high surface-to-volume ratio.6,8 With an r1 comparable to Gd-DTPA, uIONPs exhibit excellent T1 contrast for vascular imaging after intravenous administration of uIONPs in mice, and can help delineate blood vessels and enhance tumor images (Figure 2E).6 Moreover, a novel dual T1–T2 contrast effect was observed in mice, where T1 brightening contrast was observed in the hepatic vasculature while T2 (or darkening) contrast presented in the liver parenchyma (Figure 2F). This dual T1–T2 contrast effect not only opens potential clinical applications for simultaneous imaging of the vasculature and liver parenchyma, but also enables revealing tissue characteristics and locations where uIONPs accumulated. More importantly, uIONPs can be secreted through the kidney after imaging studies, improving the biocompatibility and clearance and thus the safety profile of the imaging agent. A recent study using uIONPs as imaging probes revealed that nanoparticles with sub-5 nm sizes also exhibit the enhanced permeability and retention (EPR) effect,7 one of the main driving forces for delivery of nanoparticles to tumors, and therefore improve delivery to tumors and subsequent intratumoral distribution. In this case, the bright to dark T1–T2 contrast switch caused by changing the form of uIONP dispersion in different tissue environments confirmed the delivery and tissue penetration of uIONPs, based on the appearance of bright T1 contrast followed by the uIONP clustering in the tumor after accumulation. Quantitative histological analysis confirmed that sub-5 nm nanoparticles can be delivered to the tumors much more effectively than their larger IONP counterparts.
IONPs as Magnetic Particle Imaging (MPI) Contrast Agents
Magnetic particle imaging (MPI) is an emerging, non-invasive, tomographic imaging technique that has been recently been used as a preclinical imaging tool. The MPI signal is derived from the nonlinear magnetization behaviors of IONPs with an applied magnetic field. When IONPs are present in tissue in an external magnetic field, the magnetization of IONPs can be manipulated by applying the electromagnetic energy. Because of the nonlinear magnetization behavior of IONPs, higher harmonics of the excitation frequency can be detected at the IONPs. Intensity of harmonic reflects the presence and characteristics of IONPs.11 Given that MPI signals exclusively rise from IONPs, this imaging method shows high sensitivity with little or no background, therefore offering high contrast or “hot spot” images and accurate quantification of the imaging agent. This is similar to nuclear imaging, but without the disadvantages involved in radioisotopic labels. When compared to MRI systems at typical field strengths (1.5–7 T), MPI has superb sensitivity (near pictogram of Fe) with high image acquisition rates (up to 40 volumes/second). Because the MPI signal intensity is proportional to the concentration of IONPs, it allows quantitation of IONPs at the region of interest without interference from a background signal.
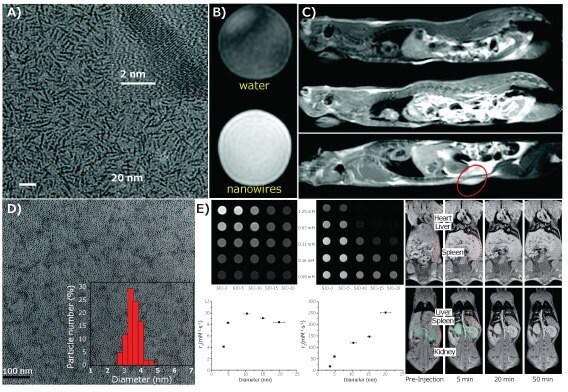
Figure 2.A) Transmission electron microscopy (TEM) image, and T1-weighted spin echo images of B) phantom and C) rat after intraperitoneal (middle) or subcutaneous (bottom) injection of iron oxide nanowhiskers, as compared to control rat (top), adapted with permission from reference 8, copyright 2018 Royal Society of Chemistry; D) TEM image of uIONPs; E) T1-, T2-weighted spin echo images, r1 and r2 values of different sized IONPs; F) Fatsuppressed T1-weighted spin echo images of a mice before and after administration of uIONPs at 10 mg·kg-1. Spleen highlighted in red dotted circles, and kidney in green dashed circles. Adapted with permission from reference 6, copyright 2014 Royal Society of Chemistry.
Among all possible design specifications for IONPs (core size, hydrodynamic size, surface chemistry, composition, biocompatibility, blood circulation time, etc.), IONP size and uniformity are the major factors in determining MPI signal strength and spatial resolution.9,10 Early work indicated that, at each operating frequency, there was an optimal core size for specific sizes of IONPs. For example, IONP with a core size of 20 nm yields optimal MPI images at 25 kHz, while 15 nm IONPs provide optimal images at 50 kHz.11 Further investigations are needed to fully understand the relationship between IONP characteristics and signal and spatial resolution. It should be noted that MRI or CT images are needed to provide anatomic information for MPI, since it only provides “hot-spots,” from where IONPs are present and does not show the structure and morphological details of tissue or organs of interest.
The first in vivo application of MPI with Ferucarbotran (Resovist®, an IONP-based MRI contrast agent with a mean hydrodynamic diameter of 60 nm) was able to show the left and right atrium, ventricle, and the pulmonary veins of the heart structure in a mouse (Figure 3A). Cell tracking and targeted imaging are two of the earliest applications of MPI. For example, neural progenitor cells labeled with Resovist® were imaged and quantified in vivo in an immunosuppressed rat model over more than 80 days. The detection limit for cell tracking studies was determined to be ~100 cells for Resovist®-labeled stem cells with current MPI systems10 In an investigation of red blood cells (RBC) migration, significant MPI signals were generated from SPION (Resovist® and Sinerem® (diameter of 20–40 nm))-labeled RBC in the high frequency range, when compared to control RBC without SPION labeling (Figure 3B–C). Interestingly, the SPIONlabeled RBC exhibited a long blood half-life, demonstrating the possibility of using MPI for real-time monitoring the blockage and impediments in circulatory systems.
MPI can be also used to visualize and track interventional medical devices and implants. In Figure 3D–E, a balloon catheter, commercially available for percutaneous intraluminal angioplasty, was filled with Resovist® tracers and inserted within a vascular phantom, and then imaged with a MPI scanner.12 With only a small amount of MPI tracer (25 mmol of iron per liter), the shaft was clearly visible with MPI, as were the balloon inflation and deflation processes, and the arterial stenosis dilation.
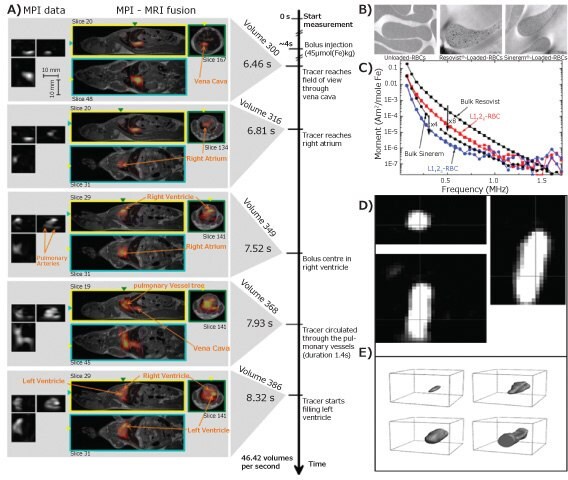
Figure 3. A) Dynamic images of MPI (left) and MPI-MRI fusion (right) of a beating mouse heart; B) TEM images of control and NP-labeled red blood cells and C) MPI signal data for Resovist®, Sinerem®, Resovist®-labeled and Sinerem®-loaded red blood cells (L1,2R-RBC and L1,2S-RBC, respectively); D) the MPI axial, coronal, and sagittal-view of balloon catheter filled with IONPs (Resovist®), and E) 3D rendering images of balloon being deflated (top left), during inflation (top right), inflated (bottom left), and being moved out of the field of view (bottom right).12
IONP-based Multimodal Imaging
Given the respective strengths and limitations of specific imaging modalities associated with the sensitivity, resolution, anatomic information, and cost, there has been a growing interest in developing multimodal imaging systems, that can combine and complement two different imaging modalities.
MRI-near Infrared (NIR) Optical Imaging
Coupling the three-dimensional, anatomically-defined deep tissue imaging capability of MRI with cost effective optical imaging and functional molecular imaging probes can provide more information about specific biological and molecular events in vivo. Furthermore, optical imaging-based surgical interventions can take advantage of the high spatial resolution and anatomic information from MRI. Among the various optical imaging probes, NIR dyes have advantages over conventional fluorescent fluorophores because of the minimal absorption interference and autofluorescence from biological samples, reduced scattering issues, and improved tissue penetration depth.
To combine NIR optical imaging with MRI, NIR dyes can be conjugated to IONPs. For example, Cy5.5 is a widely available NIR dye. It was conjugated to dextran-coated, crosslinked IONPs through amine-reactive crosslinkers, and then further modified with synthetic peptides (EPPT) as a targeting ligand to image underglycosylated mucin-1 (uMUC-1) antigens on human pancreatic adenocarcinoma. Twenty-four hours after intravenous administration of the targeting probes, a significant T2 contrast was observed within tumor areas (Figure 4A) accompanied with a high-intensity NIR fluorescence signal (Figure 4B).7 Similarly, a Cy5.5-labeled urokinase-type plasminogen activator receptor (uPAR)-targeting IONP probe was prepared to image breast tumors in mice. After conjugating the Cy5.5 and amino-terminal fragment (ATF) peptides onto IONPs, both MRI and NIR imaging were used to visualize the uPAR expressed tumors (circled in Figure 4C and 4D).4 Similarly, the use of tumor-targeting, insulin-like growth factor 1 (IGF-1) and NIR830 dye onto IONPs demonstrated the targeted MRI-NIR imaging abilities of the IGF-1 receptor positive pancreatic tumor model.5 Besides added imaging capability and information by adding a complementary modality, the NIR dye conjugated IONP, including commercially available ones (MagDye-765®, IN765-05-05) impart favorable pharmacokinetics and biomarker targeting, that are not available with low molecular weight optical imaging probes. However, the interference of IONPs with the optical properties of the original dyes, e.g., optical quenching effect, needs to be considered when combining two materials.
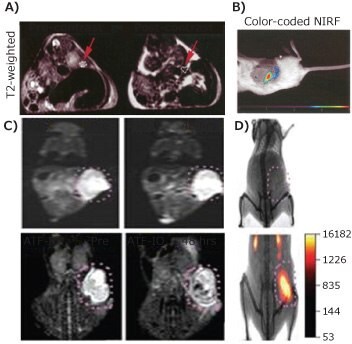
Figure 4. A) T2-weighted images and B) color-coded NIR mapping of Cy5.5-EPPT-CLIO-injected mice bearing human pancreatic adenocarcinoma, adapted with permission from reference 7, copyright 2015 American Chemical Society; C) T2-weighted MRI and D) NIR imaging of Cy5.5-ATF-IONP-injected mice bearing 4T1 mouse mammary tumors.4
MRI-Positron Emission Tomography (PET)
is the most powerful clinical molecular imaging modality. It is capable of reporting functional, molecular, and metabolic information about diseases using radioactive, positronemitting tracers. Combined PET-MRI scanners, which use MRI to visualize anatomic structures and tissue morphology, can reveal functional, molecular, and physiological information to enhance diagnosis. The combined PET-MRI is now available for clinical use. It is anticipated that as combined PET-MRI are used in more and different ways, the need for novel contrast materials that enhance both MRI and PET detection will increase. Traditional MRI-PET dual contrast agents were prepared by integrating a compound labeled with PET sensitive radioisotopes (e.g., 18F, 64Cu, 69Ge) with IONPs through chelating ligands, such as 1,4,7,10-tetraazacyclododecane-1,4,7,10- tetraacetic acid (DOTA, Cat. No. 86734) or 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA). 64Cu-bis(dithiocarbamatebisphosphonate) [64Cu-(dtcbp)2], which coupled IONPs with the PET isotope 64Cu (shown in Figure 5A) was developed to track 64Cu labeled IONPs in draining lymph nodes of mice by dual-imaging systems (Figure 5B).13 Recent advances in radiochemistry allow the synthesis of hybrid MRIPET tracers without the need for chelators. Figure 5C shows germanium (69Ge with t1/2 of 39.05 h) can be doped into IONPs during chemical synthesis by mixing 69Ge with iron precursors. This material showed excellent contrast in T2 weighted MRI and 69Ge PET.7 To obtain T1 MRI contrast agents with PET imaging capability, 68Ga was doped into dextran-coated uIONPs using a microwave-assisted route (Figure 5D), resulting in brighter T1 weighted MRI images and PET images from 68Ga-uIONPs at several iron concentrations (Figure 5E).6,14
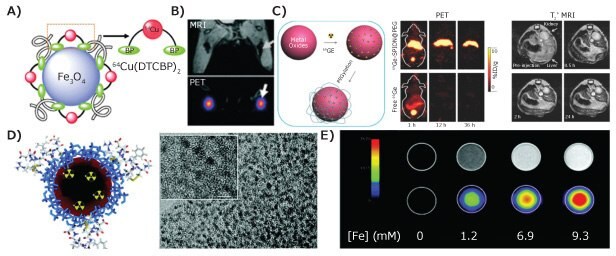
Figure 5. A) Schematic drawing of IONP-64Cu chelate; B) MRI/PET dual-mode imaging of lymph nodes with 64Cu-(dtcbp)2-IONPs, adapted with permission from reference 13, copyright 2018 Elsevier; C) schematic drawing of 69Ge-doped IONPs, and in vivo PET and T2-weighted MRI images7; D) schematic drawing and TEM image of 68Ga-uIONPs, and E) MRI phantom images (top) and PET scan (bottom) of 68Ga-uIONPs at different iron concentrations.8
Conclusions
Magnetic IONPs will remain a major platform of choice for development and application of bioimaging, for both preclinical and translational research, as well as potentially for clinical imaging. We have highlighted recent developments in the design and engineering of IONPs as contrast agents for MRI-, MPI-, and MRI-centric multimodal imaging. The growing interest in IONPs as alternatives to Gd-DTPA as a T1 MRI contrast agent will lead to further development of this new class of IONPs for clinical use. With several multi-modal imaging modalities already available for clinical applications, formulating multifunctional, hybrid IONP agents that combine contrast-enhancing effects
in multiple imaging modalities will continue to be an active area of research and development. Although it may be years before MPI is developed and approved for clinical applications, its rapid adaptation in preclinical research will be sufficient to demonstrate its capabilities and potential to expand the use of in vivo bioimaging. In the case of MPI, which requires magnetic IONPs, the development of new classes of IONPs tuned or tailored for MPI applications will be an exciting area to watch.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?