Stimuli-responsive Poly(2-isopropyl-2-oxazoline) Materials
Introduction
Various exciting phenomena exhibited by stimulus-responsive materials have greatly influenced materials design.1–3 In particular, temperature-responsive polymeric materials are used as sensors, catalysts, drug delivery systems, and separation media. Poly(2-isopropyl-2-oxazoline) (PiPrOx) is a typical temperature-responsive polymer that shows lower critical solution temperature (LCST) in an aqueous solution.4–17 At the LCST, the polymer exhibits a hydrophilic-hydrophobic phase transition. PiPrOxs have attracted attention in the biomedical field because the LCST is close to body temperature and nontoxic. Also, PiPrOxs are used as a precursor for linear poly(ethylene imine). Because the preparation of PiPrOxs proceeded by living cationic ring-opening polymerization of 2-isopropyl-2-oxazoline monomer, the average molecular weight and dispersity can be precisely controlled. The narrow molecular weight distribution of PiPrOxs shows rapid thermal transition behavior over a narrow temperature range.
Moreover, the nature of functional end groups significantly affects the LCST of PiPrOxs. The concentration of the PiPrOxs and additives such as salts or surfactants also influence the LCST. Based on the temperature properties of PiPrOxs, we have recently developed several multimodal stimuli-responsive polymeric systems. This review will briefly show recent developments of PiPrOx-based functional stimuli-responsive polymers.
Preparation and Modification of PiPrOx
Because PiPrOxs are obtained by living cationic ring-opening polymerization, various functional groups can be easily introduced to the initiation and termination ends (Figure 1). For example, propargyl-bearing clickable PiPrOxs can be prepared using propargyl tosylate as the functional initiator. Similarly, other functional groups, such as acrylate and boronic ester, can also be introduced to the initiation end. Various functional groups can be introduced by adding a nucleophilic substance to the active terminal of the living chain. For example, azide groups can be introduced to the termination end by treating the reactive chain end of PiPrOxs with sodium azide (Cat. No. 1.06688). If propargyl amine was used as a terminating agent, we could introduce a clickable propargyl group at the termination end of PiPrOxs. By treating with 4,4-bipyridyl (Cat. No. 289426), we obtained redox-active viologen-bearing PiPrOxs. The propargyl and azide groups can be further modified by click or Sonogashira coupling reaction to introduce various functional groups.
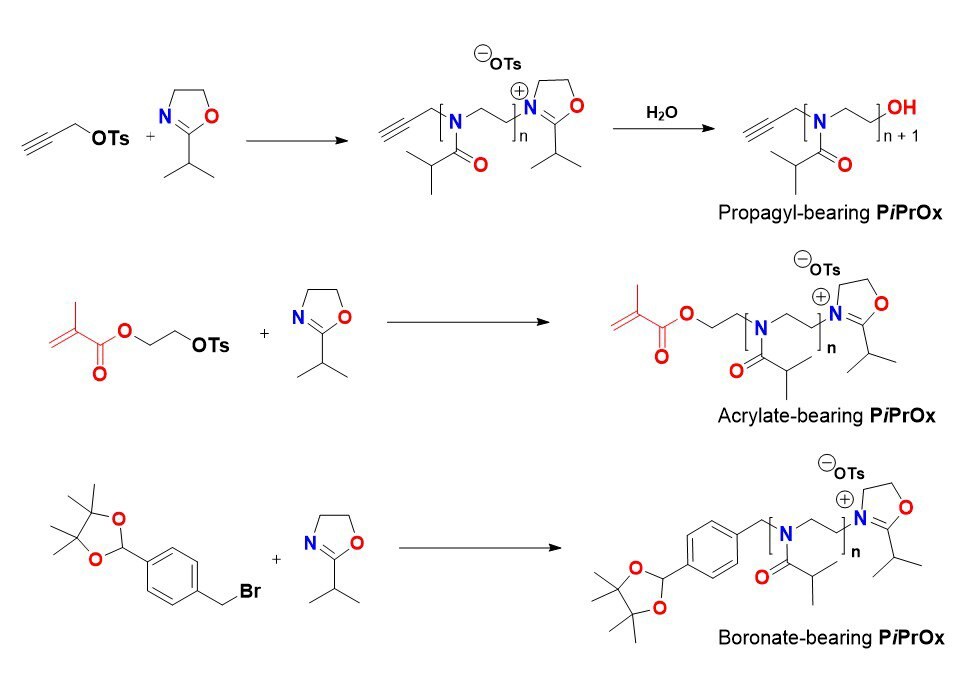
A.Functionalization of initiation end
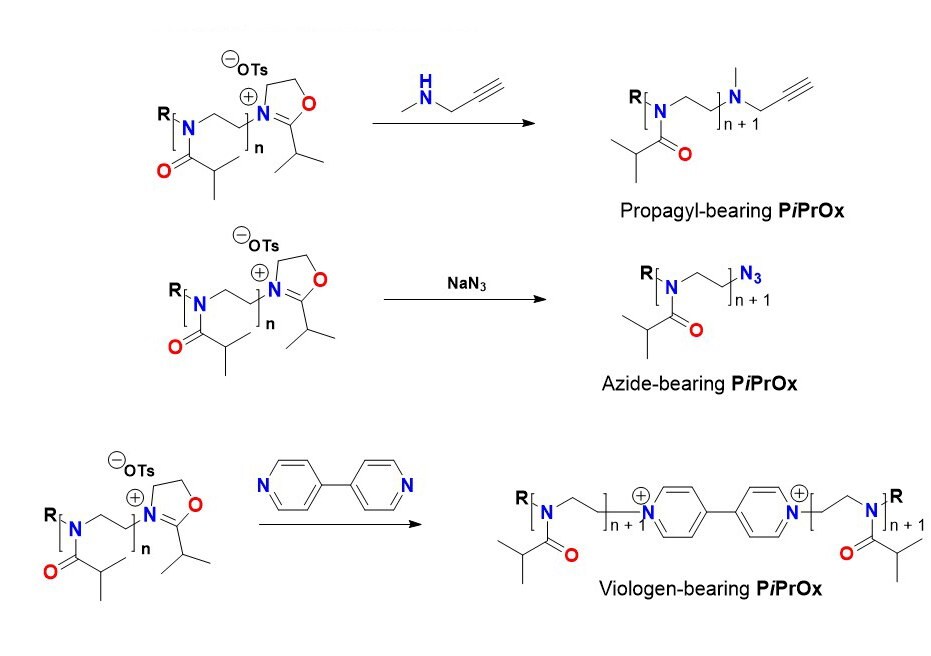
B.Functionalization of termination end
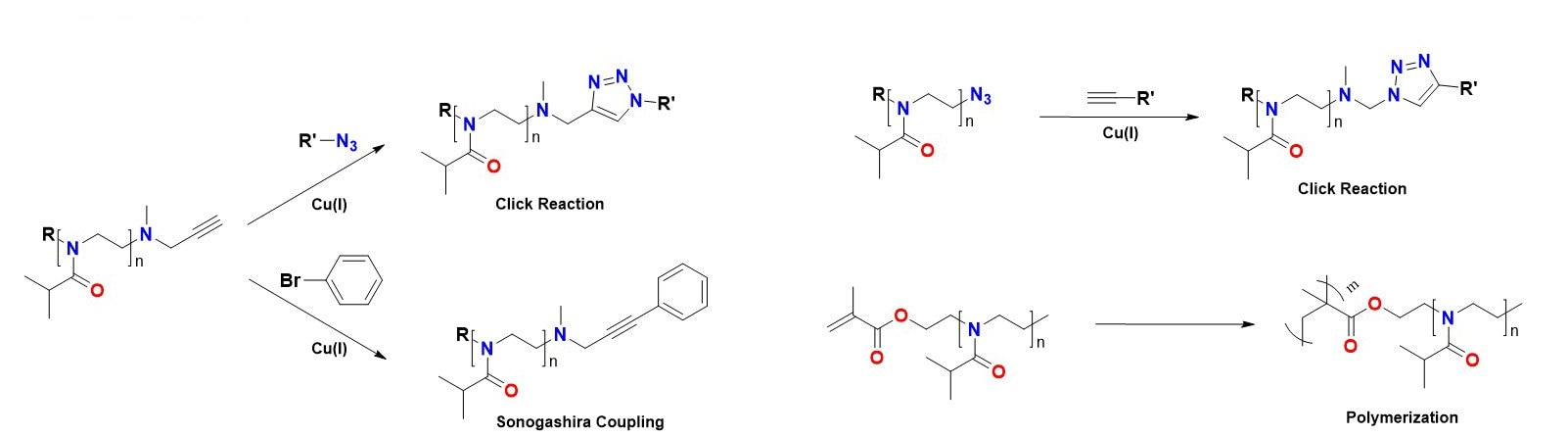
C.Modification of functional groups
Figure 1. Preparation and modification of PiPrOxs
pH Responsive Poly(benzyl ether) Dendron-conjugated PiPrOx
Dendrimers are regularly branched macromolecules that exhibit various unique properties. For example, the solution properties of dendrimers are highly dependent on the nature of surface functionalities. Poly(benzyl ether) dendrimers with peripheral benzoic acid moieties show high solubility in an aqueous medium with a neutral pH, although the benzyl ether building blocks have a hydrophobic nature. A series of dendritic-linear block copolymers (Figure 2; G2-b-PiPrOx and G3-b-PiPrOx) was synthesized as dual-mode stimuli-responsive polymers via the click reaction between azide functionalized poly(benzyl ether) dendron and propargyl-bearing PiPrOx.4,10 Although the pKa value of benzoic acid is about 4.2 in aqueous media, the pKa values of benzoic acid moieties in poly(benzyl ether) dendrimers can be further increased due to neighboring group effect. If one carboxylic acid group was deprotonated, the deprotonation of neighboring carboxylic acid could be interfered with by charge repulsion. Such effect resulted in a dramatic change of the cloud point temperature (TCP) in response to the pH changes of G2-b-PiPrOx and G3-b-PiPrOx in an aqueous solution; the TCP was recorded at a temperature corresponding to a 10% decrease in the optical transmittance by a spectrophotometer. The transmittance changes of G2-b-PiPrOx (0.05 mM) and G3-b-PiPrOx (0.05 mM) were measured in a 20 mM phosphate buffered solution (PBS, 150 mM NaCl). As the pH decreased, the peripheral carboxylates gradually protonated, and G2-b-PiPrOx and G3-b-PiPrOx were finally precipitated. As shown in Figure 2, the TCP of G2-b-PiPrOx shows changes from 35 to 65 °C between pH ranges from 5.5 to 6.9. The G3-b-PiPrOx shows more dramatic TCP changes from 35 to 83 °C between pH ranges from 5.5 to 6.5. Because the dendritic-linear block copolymers showed pH and temperature-dependent solubility changes, G2-b-PiPrOx and G3-b-PiPrOx formed different types of self-assembled structures by changing pH and temperature. For example, G2-b-PiPrOx and G3-b-PiPrOx formed fibrous assemblies at low pH and temperatures. Alternatively, a sheet-like structure was observed for G3-b-PiPrOx at high temperature and pH conditions.
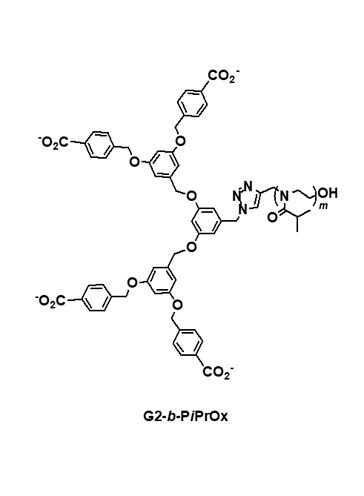
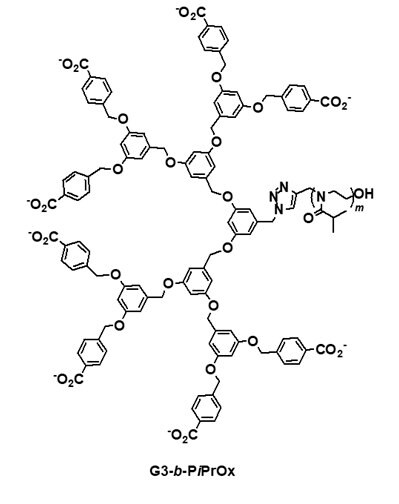
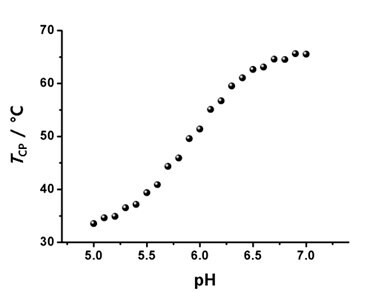
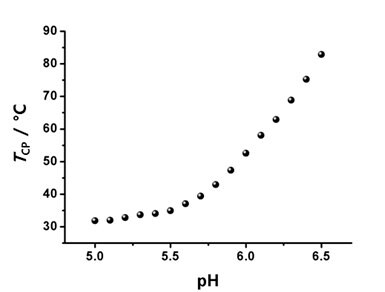
Figure 2. Structure of dendritic-linear block copolymers (G2-b-PiPrOx and G3-b-PiPrOx) with pH-dependent TCP changes. Reproduced with permission of The Royal Society of Chemistry.
Photo-responsive PiPrOx
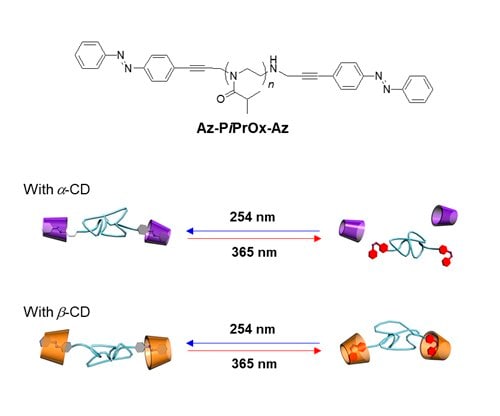
The propargyl-bearing telechelic PiPrOx was modified to azobenzene-bearing PiPrOx (Figure 3A, Az-PiPrOx-Az) via Sonogashira coupling reaction. Az-PiPrOx-Az showed reversible cis-trans photoisomerization of azobenzene units under 365 or 254 nm UV irradiation.9 Upon 365 nm UV irradiation, the Tcp of Az-PiPrOx-Az was slightly increased by photoisomerization of azobenzene groups due to the polarity change of end functional groups. The azobenzene moiety can form a host-guest complex with cyclodextrin (CD). Both trans- and cis-azobenzene moieties can be accommodated in the β-CD, but the cis-azobenzene cannot be adapted to α-CD due to its small cavity size (i.e., 0.57 nm). When the azobenzene moieties are bound to CDs, the end functional groups can be changed to a more hydrophilic state. Therefore, the Tcp of Az-PiPrOx-Az was slightly increased by the formation of host-guest complexes with α-CD and β-CD. After the formation of host-guest complexes, 365 nm UV light was irradiated to the Az-PiPrOx-Az solutions to induce trans to cis photoisomerization. As a result, the Tcp of the Az-PiPrOx-Az with α-CD and β-CD moved in opposite directions. Upon the 365 nm UV light irradiation, α-CD can be released from the host-guest complex. After photoisomerization, the β-CD with large cavities were more tightly bound to the cis-azobenzene moiety. The tight binding of cis-azobenzene to β-CD influences the Tcp of Az-PiPrOx-Az. Using these properties, we controlled the solubility of Az-PiPrOx-Az by UV irradiation at a specific temperature. The transparent solution of Az-PiPrOx-Az with α-CD at 25 °C becomes turbid under 365 nm irradiation. Alternatively, the turbid solution of Az-PiPrOx-Az with α-CD became transparent under 254 nm irradiation Az-PiPrOx-Az also achieved the opposite with β-CD at 28 °C.
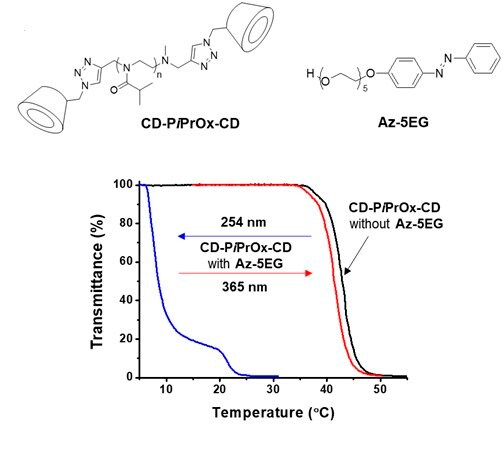
Azobenzene was used to photochemically control Tcp of PiPrOx derivatives.12 We prepared α-CD-bearing telechelic PiPrOx (Figure 3B, CD-PiPrOx-CD) as the host for azobenzene derivatives. The Tcp of CD-PiPrOx-CD (2.0 g/L-1) in 10 mM PBS solution (150 mM NaCl, pH 7.4) was displayed at 42.9 °C upon addition of the azobenzene-bearing penta(ethylene glycol) (Az-5EG) (3.3 mM) the Tcp dramatically changed to 8.5 °C. At 365 nm irradiation, the Tcp changed to 41.3 °C, indicating the release of Az-5EG. The Tcp changes were fully reversible during alternate irradiation at 365 and 254 nm.
A.
B.
C.
D.
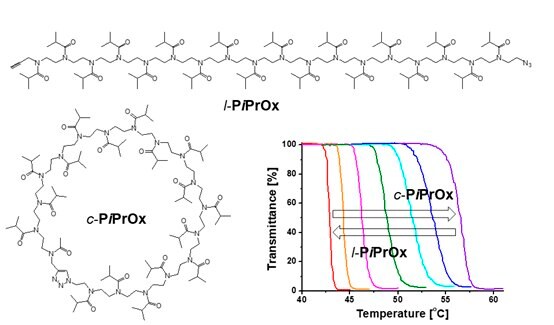
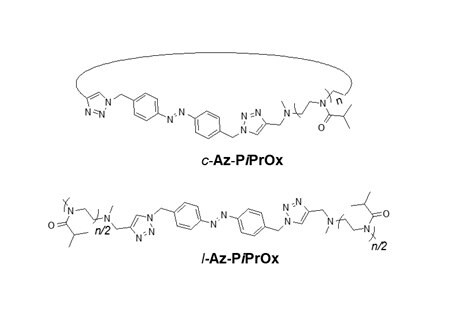
Figure 3. A) Structure of Az-PiPrOx-Az and photo-responsive host-guest complexation of Az-PiPrOx-Az with cyclodextrins. B) Structures of CD-PiPrOx-CD and Az-5EG with photo-responsive temperature-dependent transmittance changes. C) Structures of l-PiPrOx and c-PiPrOx with temperature-dependent transmittance changes of l-PiPrOx and c-PiPrOx mixtures. D) Structures of l-Az-PiPrOx and c-Az-PiPrOx. Reproduced with permission of The Royal Society of Chemistry and The American Chemical Society.
Cyclic PiPrOx
Because propargyl and azide groups can be introduced to the initiation and termination ends, cyclic PiPrOx (Figure 3C, c-PiPrOx) can be prepared through an intramolecular click reaction.6 When compared to linear PiPrOx (Figure 3C, l-PiPrOx) with the same molecular weight and dispersity, c-PiPrOx showed much higher Tcp. The Tcp of l-PiPrOx displayed photosensitivity at 43.4, 45.9, and 50.6 °C for 5.0, 3.0, and 1.0 g/L, respectively. In the case of c-PiPOx, the Tcp appeared at 56.3, 60.2, and 69.0 °C for 5.0, 3.0, and 1.0 g/L, respectively. When the c-PiPrOx and l-PiPrOx were mixed, the Tcp was gradually increased as the contents of c-PiPrOx increased. Using this property, we could precisely control the thermal transition temperature by changing the mixing ratio.
We also prepared azobenzene-containing linear and cyclic PiPrOxs (Figure 3D, l-Az-PiPOx and c-Az-PiPOx, respectively) to control Tcp photochemically.7 As expected, the photoisomerization of the azobenzene moiety resulted in the change of the Tcp of the polymers. Cis isomers exhibited a higher thermal transition temperature than trans isomers.
Fluorescent Dye-conjugated PiPrOx
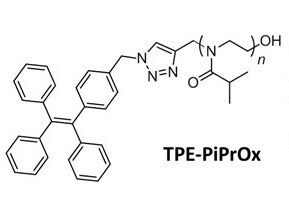
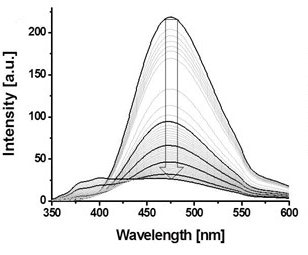
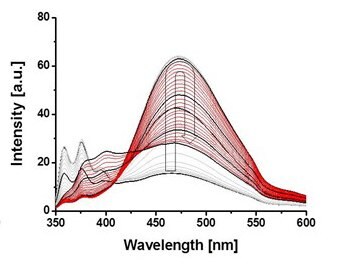
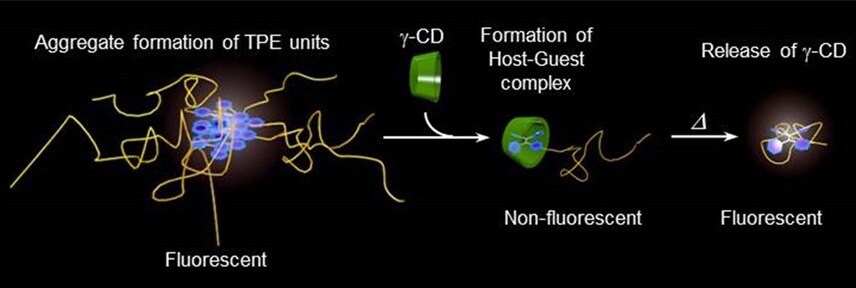
Tetraphenylethene (TPE), a well-known aggregation-induced emission (AIE) unit, was introduced to PiPrOx (Figure 4, TPE-PiPrOx) through the click reaction between azide-bearing TPE and propargyl-bearing PiPrOx.11 The TPE-PiPrOx exhibited strong fluorescence due to the formation of a micellar structure in an aqueous solution. As the temperature increased, the fluorescence intensity of TPE-PiPrOx gradually decreased due to the thermal relaxation of the excited state. Interestingly, the fluorescence emission of TPE-PiPrOx completely disappeared with the addition of γ-CD, indicating inclusion of the TPE unit into the cavity of γ-CD. The emission of TPE-PiPrOx with γ-CD increased by thermal transition of the the PiPrOx chain. Above Tcp, the hydrophobic PiPrOx chain could be associated with TPE units to expel γ -CD from TPE-PiPrOx.
A.
B.
C.
D.
Figure 4. A) Structure of TPE-PiPrOx, B) fluorescence emission (λex = 310 nm) spectra of TPE-PiPrOx (50 μM in 10mM PBS, pH 7.4) and C) TPE-PiPrOx with γ-CD (20 eq) upon heating from 25 to 70 °C. D) The picture describes the host-guest complex of the TPE-PiPrOx with γ-CD and thermal transition. Reproduced with permission of The Royal Society of Chemistry.
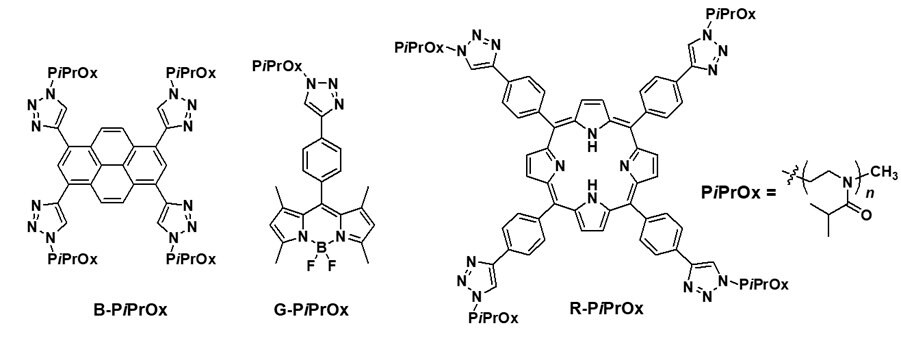
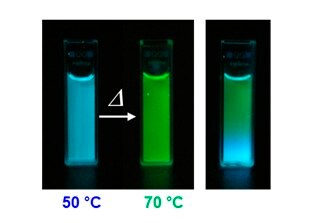
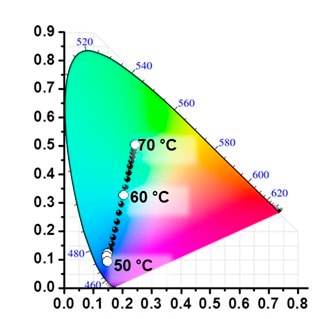
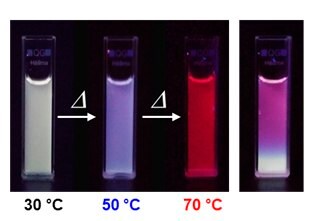
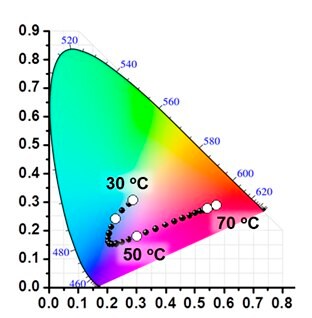
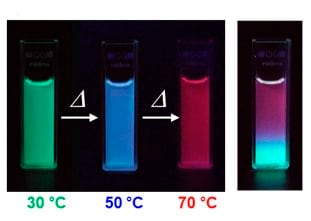
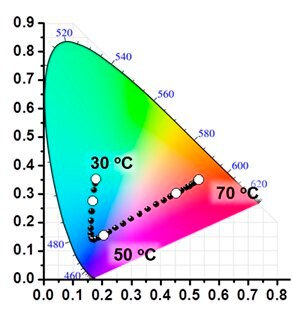
Three primitive color-emitting dyes, pyrene, boron-dipyrromethene (BODIPY), and porphyrin, were conjugated with azide-bearing PiPrOxs to obtain Dye-PiPrOxs (Figure 5, B-PiPrOx, G-PiPrOx, and R-PiPrOx).8 Each Dye-PiPrOx exhibited strong fluorescence emission in the aqueous phase. The Tcp of B-PiPrOx, G-PiPrOx, and R-PiPrOx appeared around 47, 43, and 39 °C, respectively. Above Tcp, the emission color of B-PiPrOx was slightly changed from blue to greenish white due to the partial formation of excimer. On the other hand, the emission of G-PiPrOx was completely quenched above Tcp. Although the emission intensity of R-PiPrOx gradually decreased by increasing temperature, the red emission remained about 20% even above Tcp. Since Dye-PiPrOxs emit three primary colors, a variety of colors can be created by combining them. Moreover, the emission color was varied by temperature changes. For example, the mixture solution of B-PiPrOx (1.0 × 10-6 M) and G-PiPrOx (1.0 × 10-6 M) showed blue emission at room temperature. As temperature increases, the mixture solution's blue emission gradually changes to green color. Moreover, both blue and green colors appeared from the solution under temperature gradient. The ternary mixture solution of B-PiPrOx (0.1 × 10-6 M), G-PiPrOx (0.5 × 10-6 M), and R-PiPrOx (5 × 10-6 M) initially showed white emission which gradually changed to blue and red. When the mixing ratio was changed to B-PiPrOx (0.5 × 10-6 M), G-PiPrOx (4 × 10-6 M), and R-PiPrOx (3 × 10-6 M), the initial green emission gradually changed to blue and finally changed to red.
A.
B.
E.
C.
F.
D.
G.
Figure 5. A) Structures of Dye-PiPrOxs. Emission color changes of Dye-PiPrOx mixture systems: B, E) B-PiPrOx (1.0 × 10-6 M) and G-PiPrOx (1.0 × 10-6 M); C, F) B-PiPrOx (0.1 × 10-6 M), G-PiPrOx (0.1 × 10-6 M), and R-PiPrOx (5 × 10-6 M); and D, G) B-PiPrOx (0.5 × 10-6 M), G-PiPrOx (4 × 10-6 M), and R-PiPrOx (3 × 10-6 M). Reproduced with permission of The Wiley-VCH.
Chemo-responsive PiPrOx
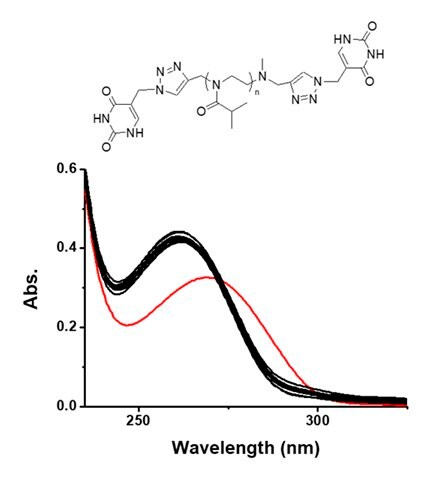
An uracil-bearing telechelic PiPrOx (Figure 6, U-PiPrOx-U) was synthesized by click reaction between propargyl-bearing PiPrOx and uracil having an azide group.14 To U-PiPOx-U, a total 23 kinds of metal ions were added, and the absorption change of the solution was monitored. As a result, only Hg2+ caused bathochromic absorption shift of the U-PiPOx-U, indicating the selective binding of Hg2+ to uracil moiety. The Tcp of U-PiPOx-U (0.8 g L-1) was appeared at 58.1 °C, which gradually decreased to 37.8 °C by addition of Hg2+.
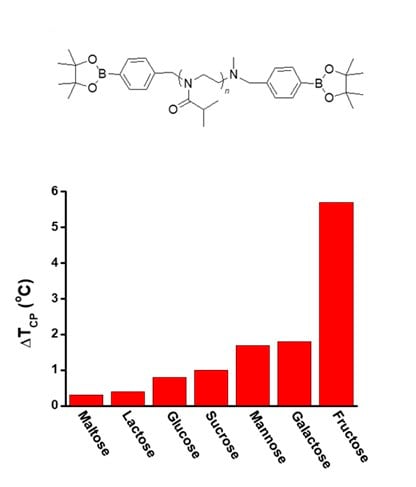
As another type of chemo-responsive PiPrOx, a pinacol boronate-bearing telechelic PiPrOx (Figure 6, B-PiPrOx-B) was prepared.13 Since boronic esters can be exchanged with chemicals having diol groups, the Tcp change of B-PiPrOx-B was investigated in the presence various saccharides such as maltose, lactose, glucose, sucrose, mannose, galactose, and fructose. As a result, the Tcp of B-PiPrOx-B increased with the addition of various saccharides. However, the degree of Tcp changes greatly depended on the saccharides, where the highest binding affinity has been observed for fructose. Because the solubility of B-PiPrOx-B can be controlled by adjusting the fructose concentration, the design of a fructose-mediated delivery system would be feasible.
Redox-responsive PiPrOx
A viologen-containing thermo-responsive PiPrOx (Figure 6, PiPrOx-V) was synthesized by treating the active terminal of living polymer with 4,4-bipyridyl.15 An aqueous solution of PiPrOx-V showed decreasing transmittance by increasing temperature to 50 °C through the hydrophilic-hydrophobic phase transition. With the addition of sodium dithionite, the transparent solution of PiPrOx-V immediately turned blue through the formation of a radical cation by single-electron reduction. The blue color gradually disappeared by aerobic oxidation. Along with the color change, the Tcp of PiPrOx-V was also changed by the redox state change of the viologen unit. Because PiPrOx-V exhibited reversible redox behavior, electrochromic changes were investigated using the sandwich-type ITO glass cell containing an aqueous solution of PiPrOx-V (180 mg/mL) with potassium ferricyanide (20 mM) as an electrolyte. Upon 1.5 V of electric potential applied to the ITO glass cell, the solution turned to violet phase. The violet phase was reversibly changed to the transparent phase when the applied voltage was removed. The PiPrOx-V-containing ITO glass cell exhibited four different visual states with changes in temperature and electric potential. The thermal and electrochromic changes of PiPrOx-V were fully reversible for 100 cycles of repeated voltage shifts.
A.
B.
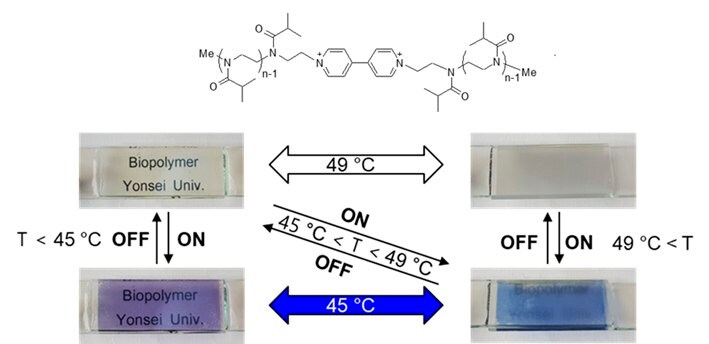
C.
Figure 6. A) Structure of U-PiPrOx-U with UV-Vis absorption changes upon addition of various metal ions (Hg2+, Ag+, Al3+, Au+, Ba2+, Ca2+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Fe3+, K+, Li+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Pt2+, Rh2+, Ru3+, and Zn2+). B) Structure of B-PiPrOx-B with Tcp changes by addition of various sugars. C) Structure of PiPrOx-V with phase transition behaviors by temperature and electric potential changes. Reproduced with permission of The Royal Society of Chemistry.
Conclusion
We have briefly introduced several PiPrOx-based functional stimuli-responsive polymeric materials. Since various functional groups can be introduced to both initiation and termination ends of PiPrOx, we could easily combine other types of stimuli-responsive functions with thermo-responsiveness. Moreover, the introduction of propargyl or azide end groups are beneficial for further modification of the PiPrOx. The sharp hydrophilic-hydrophobic thermal transition of PiPrOx is a strong motif for functional polymeric materials and devices. Although most of the examples introduced in this review simply show examples of responding to two different stimuli at the same time, it will be possible to design materials that exhibit structural and functional changes in response to a more significant number of stimuli simultaneously.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?