Triphenoxazoles: Fluorescent Photoconducting Materials
Contents
- Introduction
- Triphenoxazoles
- Photophysics
3.1. Absorbance and Fluorescence
3.2. Photoconduction - Material Self-Assembly
4.1. Liquid Crystallinity
4.2. Nanowire Self-Assembly - Incorporation into Polymers
5.1. 3D Printing
5.2. Spin Coating - Chemo-Sensing
6.1. Protons
6.2. Cations
6.3. Electron Poor Aromatic - Bio-Imaging: Multiphoton Imaging
- ChromaTwist Triphenoxazole Materials Currently Available
1. Introduction
Extended organic π-molecular structures have interesting photo-physical1, 2 and electro-physical3, 4 properties. Their study has led to the academic disciplines of organic electronics5 and plastic electronics,6 which are used in OPVs7, 8, OLEDs,9 and fluorescent (bio)imaging reagents.10
Molecular electron donor-acceptor based organic materials exhibit tuneable electronic characteristics11 by modification of the donor and/or acceptor moiety, leading to, for example, Stokes shift modification.12 However, in molecular-based materials the degree of tunability has been relatively small when compared to organo-metallic complexes,13, 14 inorganic materials15, 16 and conjugated organic polymers.17, 18 The HOMO and LUMO of such materials can be manipulated to a greater degree than molecular based materials, leading to the modification of Stokes shifts, for example.19 Thus, molecular-based materials with such properties are highly prized.
Here we introduce the Triphenoxazoles (Figure 1), a new class of organic electron donor-acceptor materials that can both be manipulated to change the electronic properties of the donor and acceptor with the consequent modulation of their HOMO/LUMO and properties, such as Stokes shift and (photo)conductivity, whilst at the same time displaying liquid crystallinity and functional groups for further chemical modification.
2. Triphenoxazoles
The Triphenoxazoles are based on the liquid crystalline hexapentyloxytriphenylene (HPT) core to which an oxazole moiety has been fused with alky and aryl groups (R) grafted to the C2 oxazole position. The nature of this R group modulates the chemical, material and electro-optic properties of the Triphenoxazoles.
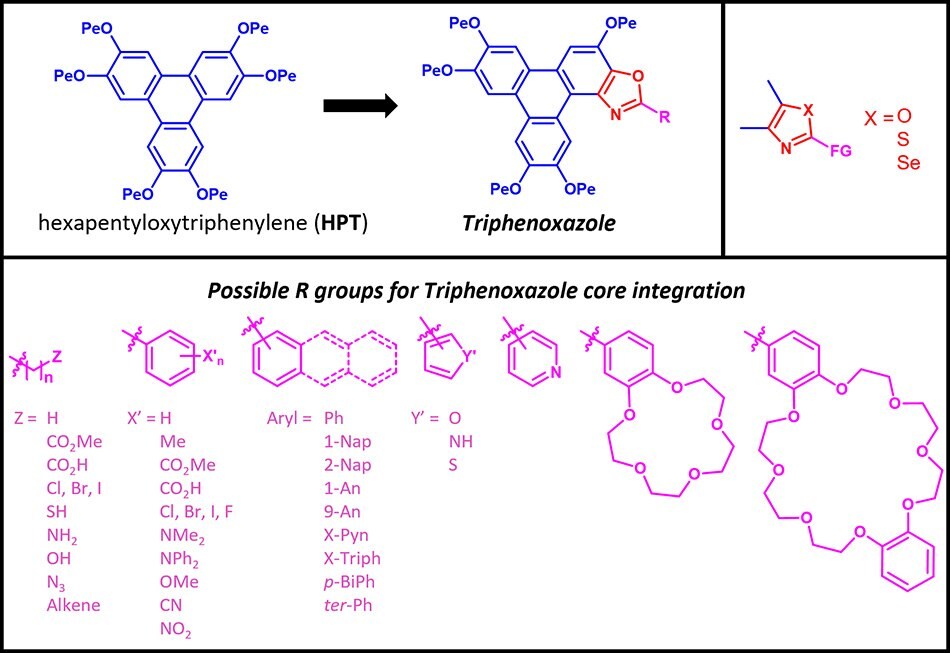
Figure 1.A Selection of Triphenoxazoles
In the following sections, we explore the physics and material properties of the Triphenoxazoles.
3. Photophysics
3.1 Absorbance and Fluorescence
The HPT and TpOx-n-Bu absorption spectra are remarkably similar (Figure 2a). The substitution of the Bu moiety (TpOx-n-Bu) with the phenyl group (TpOx-Ph) shifts the λmax blue by 3 nm to ~271 nm, but extends the absorption to the red significantly. This behavior is replicated for the higher linear extended aryl analogs (1-Nap, 2-Nap, and 2-An (Figure 2b, c), whereas the 9-An derivative’s λmax is no longer the band at ~271 nm, but ~252nm.
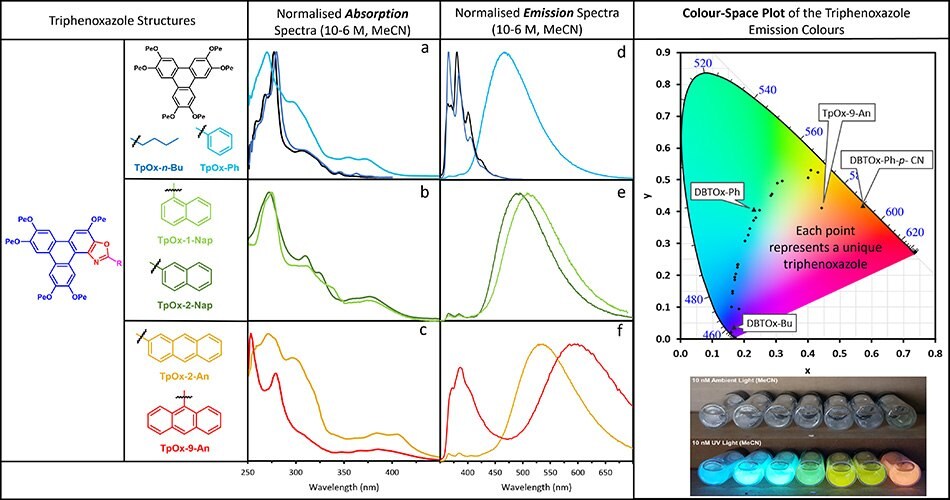
Figure 2.The absorption (a-c) and emission (d-e) spectra of a selection of Triphenoxazoles. The color space plot (top right), and solutions under ambient and UV light (bottom right).
The fluorescence spectra of HPT and TpOx-n-Bu are similar, but with different λmax values, 381 and 366 nm, respectively (Figure 2d). The substitution of the butyl group (TpOx-n-Bu) with the phenyl moiety (TpOx-Ph) has a profound effect on the fluorescence spectra, both in terms of increased peak width and red shift of the emission λmax by ~100 nm to 467 nm. Further extension of the aryl appendage to naphthalene and anthracene moieties shifts the emission λmax even redder, with 9-An having – what is believed to be – the largest Stokes shift (341 nm, 22690 cm-1) for a purely organic molecular material. This photophysical data is summarised in Table 1.
The HOMO and LUMO values for selected TpOx-R derivatives are shown in Figure 3 (together with 3 related structure DBTOx-R structures). The HOMO energy remains constant and similar to the HPT, whereas the LUMO energies decrease as a function of the type of appendage on the TpOx moiety. This reduction in LUMO indicates that the band gap decreases as the aryl appendage π-surface area increases from phenyl (TpOx-Ph) to naphthyl (TpOx-2-Nap) to anthracyl (TpOx-9-An), in line with the fluorescent red shift across the TpOx-Ar series.
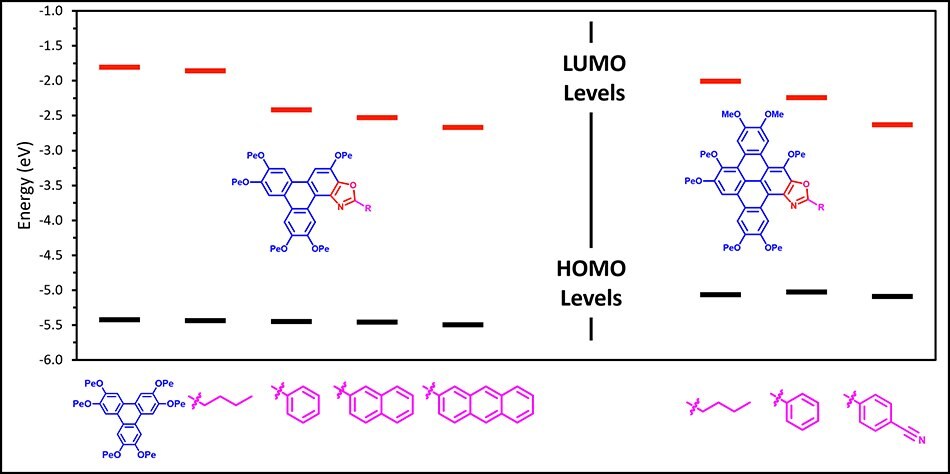
Figure 3.The HOMO and LUMO values of selected Triphenoxazoles (left) and their DBTOx-R (right) derivatives.
3.2 Photoconduction
The photocurrent measurements (Figure 4) reveal that TpOx-Ph display a photocurrent some 50-fold greater than HPT and TpOx-n-Bu. TpOx-1-Nap and TpOx-2-Nap then increase significantly over TpOx-Ph. However, the TpOx-2-An photocurrent reduces significantly back to the same levels as TpOx-Bu, presumably due to different packing arrangements in the condensed state.
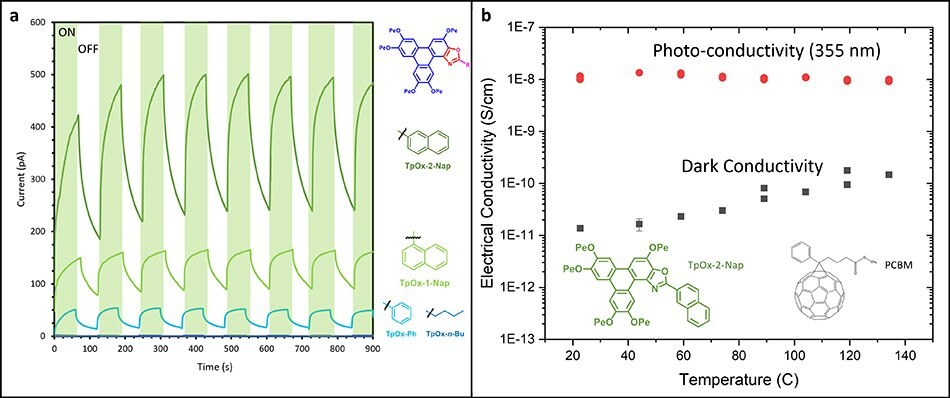
Figure 4.(a) The photocurrent behavior of selected Triphenoxazoles, and (b) the photo-conductivity response of an TpOx-2-Nap and PCBM formulation.
4. Material Self-Assembly
4.1 Liquid Crystallinity
The optical polarised microscopy image (OPM) and X-ray diffraction pattern of TpOx-n-Bu are shown in Figure 5a and 5b, respectively. Both measurements are indicative of hexagonal columnar ordering similar to the parent HPT. The OPM textures of the TpOx-Ar derivatives in the liquid crystalline phase are all similar to TpOx-n-Bu (except for TpOx-9-An which does not display a mesophase). Therefore, it is assumed TpOx-Ar derivatives form mesophases which are columnar hexagonally ordered (except TpOx-9-An). The phase ranges are summarised in Figure 6c (red bar).
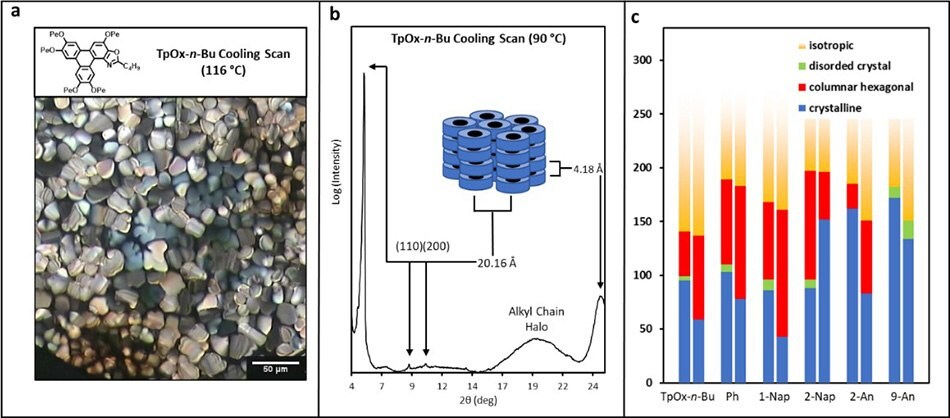
Figure 5.(a) Optical polarised image and (b) XRD of TpOx-n-Bu in its liquid crystalline phase, and (c) a summary of the phase behaviour of selected TpOx-R derivatives.
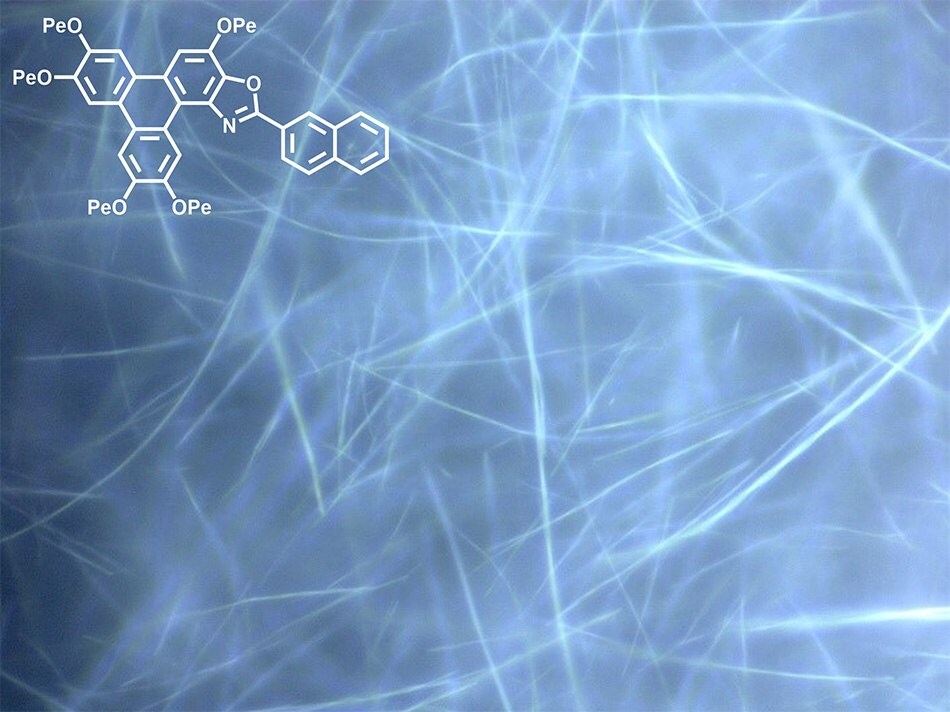
Figure 6.Nanowires (100-300 nm diameter) formed from TpOx-2-Nap via self-assembly from solution.
5. Incorporation of TpOx-R into Polymer Matrices
This section describes two methodologies that have been used to incorporate the TpOx-2-Nap into a resin and PMMA, to show how the TpOx-Rs can be 3D printed and spin coated, respectively, for potential use in plastic/flexi-electronic applications.
5.1 3D Printing
The TpOx-2-Nap was incorporated into a 3D printable resin and printed into a disc. The disc maintained fluorescent (Figure 7a), revealing the TpOx-Rs are amenable to relatively high-temperature processing. Indeed TGA analysis of the TpOx-Ar derivatives reveals thermal stability up to at least 250 oC (Figure 7b).
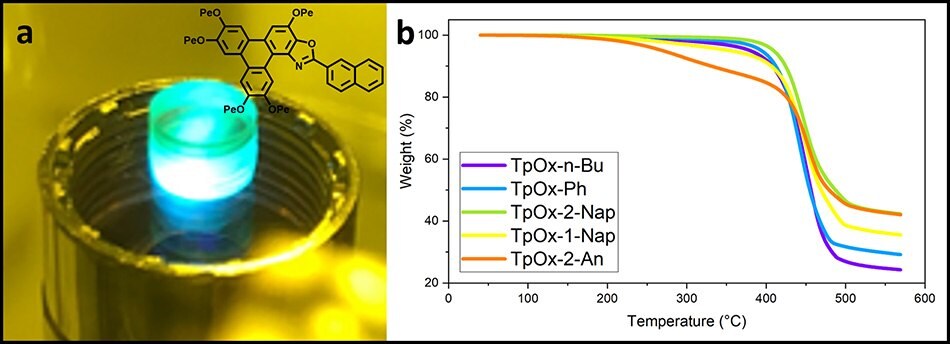
Figure 7.(a) A 3D-printed disc with TpOx-2-Nap doped resist and (b) the TGA curves of the TpOx-Ar derivatives showing the thermal stability. (Figure 7a courtesy of Dr Simon Leigh, University of Warwick).
5.2 Spin Coating
Solutions of PMMA incorporating 0.05wt% - 10wt% of TpOx-2-Nap were spin coated to a film thickness of ~480 nm. Film fluorescence was observed across all concentrations (Figure 8a). As the doping concentration increased from 0.05wt% to 2wt%, there was a red shift in the emission λmax from 467 nm to ~490 nm, after which the fluorescence λmax remained constant at ~490 nm (Figure 8b), which was ~4nm bluer than the solution-based fluorescence.
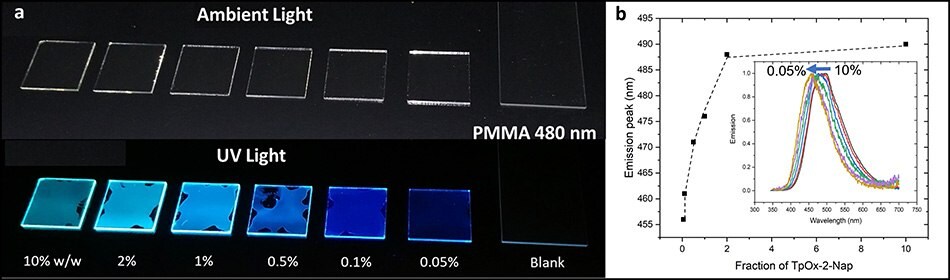
Figure 8.(a) Spin coated PMMA films doped with TpOx-Ph and (b) their fluorescence spectra revealing emission dependence on the TpOx-2-Nap doping level.
6. Chemo-Sensing
The Triphenoxazoles fluorescent properties makes them interesting materials for chemo-sensors, by either:
- modulating the fluorescent colour, or
- switching off the fluorescence when exposed to an analyte.
Below we report three different systems that are sensitive to protons, cations and π-electron poor aromatics.
6.1 Protons
TpOx-Ph-m-NMe2 has a protonatable aromatic amine as the aryl appendage. In the unprotonated neutral state, the -NMe2 moiety will act as a π-electron donor into the phenyl moiety, whereas once protonated the ammonium cation will act as an inductively coupled electron withdrawing (-I) group, and hence modify the electronics of the aryl appendage significantly. This protic modification and the fluorescent response were probed via a trifluoroacetic acid titration (Figure 9). The emission λmax of unprotonated TpOx-Ph-m-NMe2 (457 nm) was red shifted as the pH fell, plateauing at ~520 nm.
![TpOx-Ph-m-NMe2 emission modulation TpOx-Ph-m-NMe2 emission modulation as a function of [H+].](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/materials-science-and-engineering/biosensors-and-imaging/triphenoxazoles/tpox-ph-m-nme2-emission-modulation/tpox-ph-m-nme2-emission-modulation.jpg)
Figure 9.TpOx-Ph-m-NMe2 emission modulation as a function of [H+].
6.2 Cations
TpOx-B15C5 was designed to introduce a cation binding motif on to the aryl appendage, via the bezo-15-crown-5 crown unit. Several metal cations were added to a solution of TpOx-B15C5 (Fluorescence λmax = 470 nm) which saw red shifts upon addition of the Li+ and Mg2+ cations to ~480, and 490 nm, respectively (Figure 10).
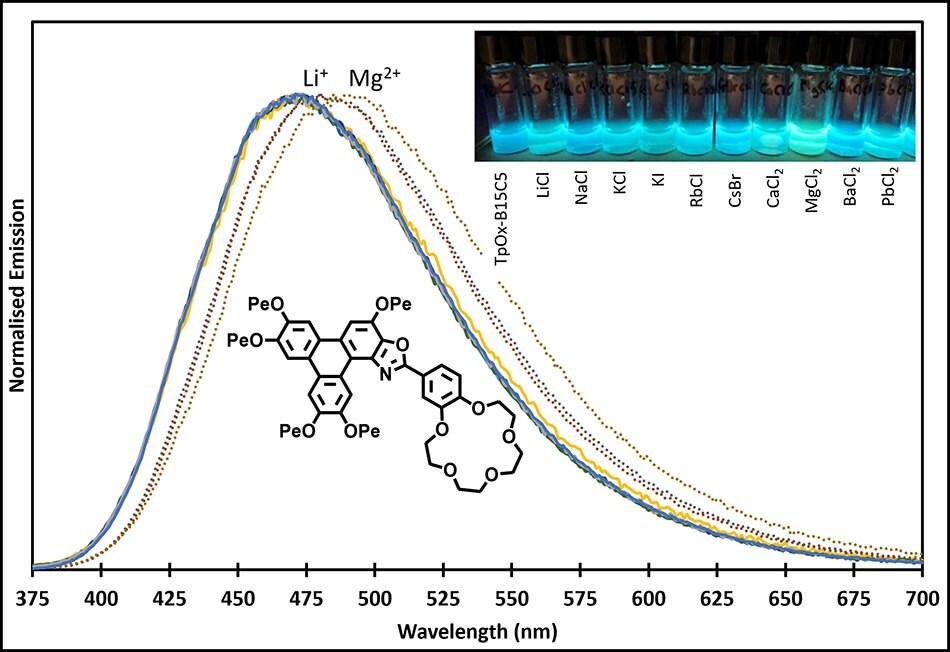
Figure 10.TpOx-B15C5 emission response and solutions under UV light to several cations.
6.3 Electron Poor Aromatics
The sensing of electron poor aromatics is important, as compounds, such as TNT, are explosives. Thus, experiments were performed to examine the sensitivity of the TpOx-Ars to the electron poor nitro-benzyl alcohol (Figure 11). In all cases, the fluorescence was quenched upon addition of the nitro-benzyl alcohol.
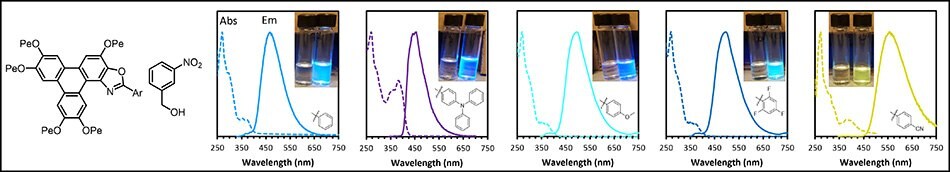
Figure 11.TpOx-Ar emission response to 3-nitro-benyzl alcohol.
7. Bio-Imaging: Multiphoton Microscopy
A aqueous perfusate of TpOx-2-Nap (1mg mL-1) was perfused into an ex-planted liver and interrogated with multiphoton microscopy using an 825 nm excitation source. Figures 12a and b show that not only have the hepatocytes (stained with a commercial red membrane stain) been infiltrated by the TpOx-2-Nap (diffuse green fluorescence), but also the T-cells coursing through the vasculature have also been infiltrated (bright green fluorescence, Figure 12a)
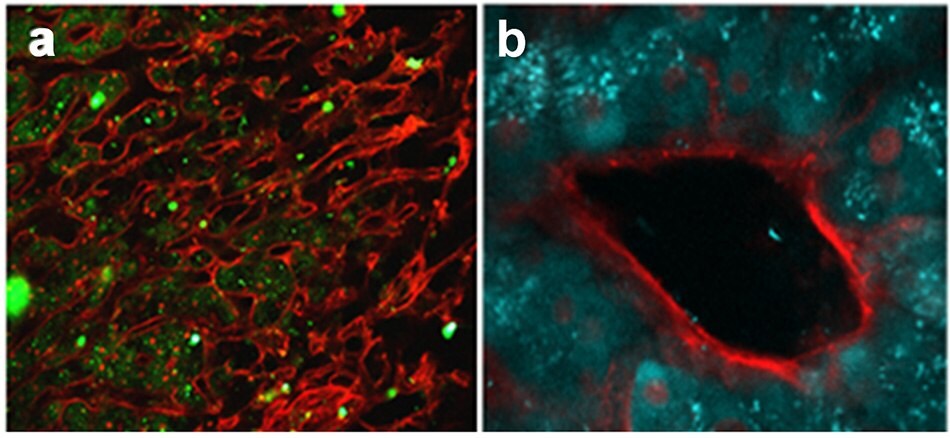
Figure 12.(a) Multiphoton microscopy images of liver section perfused with TpOx-2-Nap, and (b) image of liver vasculature made possible by the retention of TpOx-2-Nap in the hepatocytes. (Figures courtesy of Dr Zania Stamataki and Dr Scott Davies, University of Birmingham).
8. ChromaTwist Triphenoxazole Materials Available from Merck
Figure 13 provides an overview of ChromaTwist Triphenoxazole materials available for purchase. Tables 2 and 3 list their photophysical and liquid crystalline properties. The 18 data sheets reveal the absorption and emission spectra, together with an image of the fluorescence colour in solution (ethyl acetate) and the optical polarising microscopy image of each material in its liquid crystalline phase.
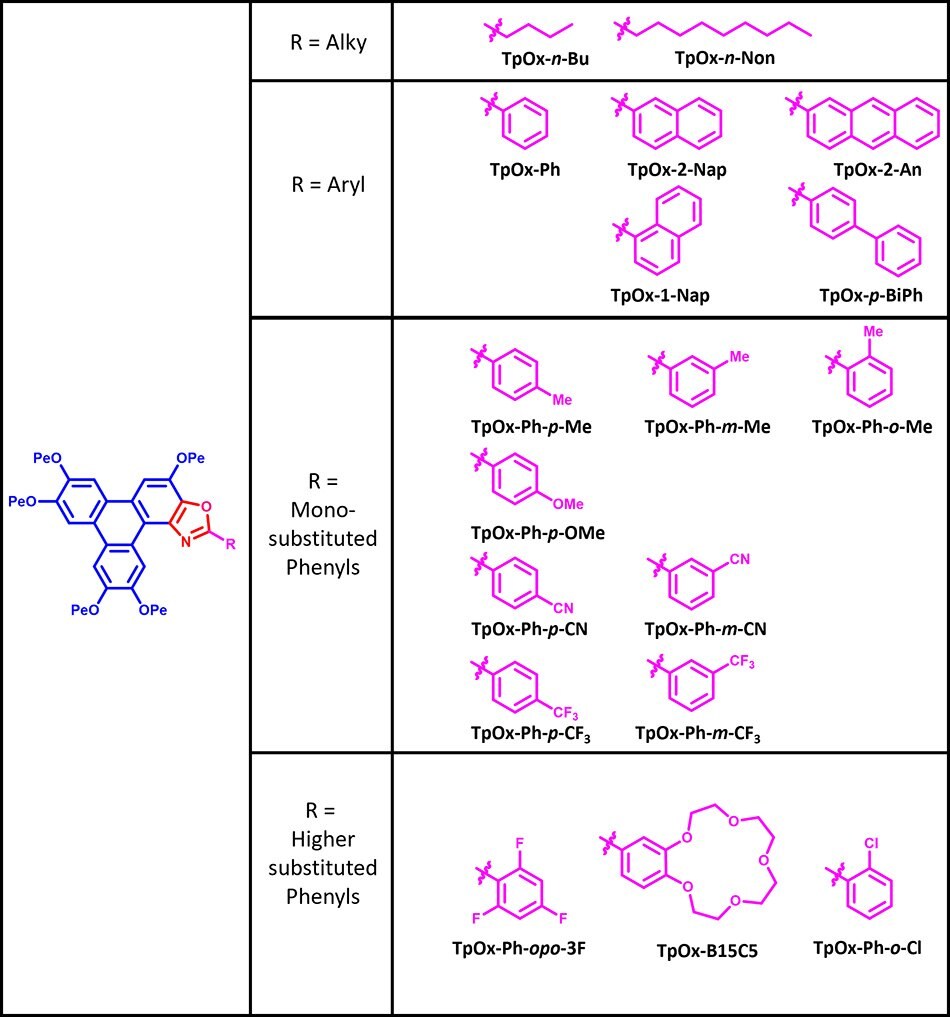
Figure 13.Available Triphenoxazoles materials.
Triphenoxazoles Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?