Synthesis of Polyamino Acids
Introduction
Polyamino acids are able to adopt ordered conformations, such as α-helices and β-sheets, through cooperative hydrogen bonding. These conformations impart polyamino acids with various unique properties and functions in biological environments. The development of controlled ring-opening polymerization (ROP) of α-amino acid N-carboxyanhydrides (NCAs) in the past two decades has enabled the synthesis of a large quantity of polyamino acid materials with predictable molecular weight (Mw) and narrow molecular-weight distribution (polydispersity or PDI).1, 2 The innate ability of polyamino acids to adopt functionally ordered conformations in conjunction with the capability of highly controlled synthesis in large scale has expedited the widespread use of this class of materials, especially in the fields of drug delivery, tissue engineering, catalysis, and self-assembly.3, 4
Polyamino acids are the first class of biomaterials used as nonviral gene delivery vectors,5 among which cationic poly-l-lysine (PLL) is the most widely studied one. While capable of binding and condensing anionic DNA, PLL and its derivatives generally display low transfection efficiencies and, therefore, are largely abandoned in favor of polymers such as polyethyleneimine (PEI).6‑9 Despite the inability of PLL to function as a stand-alone vector, polyamino acids have been adopted as components to increase the delivery efficiency of other gene vectors. In particular, cell penetrating peptides (CPPs) such as Penetration10 and Tat (HIV Tat-derived peptide with the sequence of RKKRRQRRR)11 have found use as membrane-active ligands incorporated into existing delivery vectors to promote cell internalization, endosomal escape, and accordingly increase the transfection efficiency.12 Helical conformation is often observed in CPPs or formed in CPPs during membrane transduction, and has been closely tied to their membrane activity.13 Due to their short length and insufficient cationic charge density, CPPs lack the capability to mediate gene delivery on their own. Therefore, it is of great interest in the design and synthesis of polyamino acid vectors that possess the structural characteristics of CPPs (i.e., helical secondary structure) with adequate length and cationic charge density to function as standalone vectors.
Herein, we report our efforts on the development of a new polymerization method of NCAs, design and synthesis of watersoluble α-helical ionic polyamino acids, and the application of these new polyamino acids in gene delivery wherein the secondary structure plays a crucial role.
Synthesis of Polyamino Acids Using Organo–Silicon Initiators
In 2007, we developed a controlled ROP system for various NCA monomers using hexamethyldisilazane (HMDS) as an initiator.14 Compared with traditional amine initiators, HMDS allows better control over MW and MWD, and enables the preparation of well-defined block co-polyamino acids. A unique trimethylsilyl carbamate (TMS-CBM) moiety was identified as the chain propagating group to achieve the controlled polymerization of NCA (Scheme 1A). The mechanism of the polymerization, differing from the traditional amine-initiated “amine mechanism” and “activated monomer mechanism,” resembles the group transfer polymerization (GTP) for methyl methacrylate.15 In addition, this metal-free living polymerization can be expanded to various N-trimethylsilyl (N-TMS) amines and allows the facile functionalization of C-termini of polyamino acids (Scheme 1B).16 A variety of functional groups including alkene, alkyne, and norbornene were easily introduced into polyamino acid chains, which served as reactive sites for further chemical modification. For example, this system can be integrated with ring-opening metathesis polymerization (ROMP) to produce well-defined polyamino acids containing brush17 or block polymers18 by using either N-TMS amine-functionalized ROMP monomers or chaintransfer agents (CTAs). These novel hybrid copolymers bearing intrinsic secondary structures have shown interesting patterns and supramolecular architectures in self-assembly.19
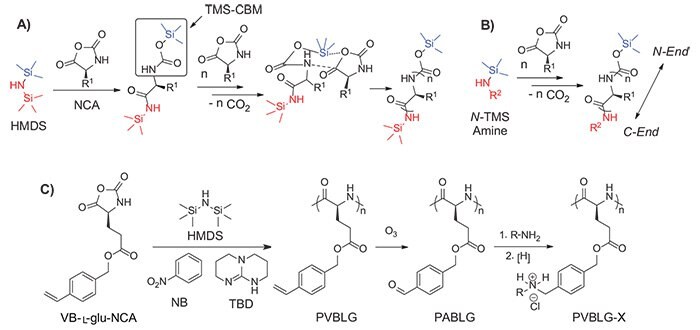
Scheme 1. A) Proposed mechanism of hexamethyldisilazane (HMDS) initiated controlled NCA polymerization. Trimethylsilyl carbamate (TMS-CBM) moiety was identified as the chain propagating group. B) Synthesis of C-termini functionalized polyamino acids initiated by N-TMS amines. C) Synthesis of water-soluble α-helical polyamino acids PVBLG-X.
In addition, we also developed novel NCA monomers to enable side-chain manipulation of polyamino acids. NCA monomers bearing vinyl groups [e.g., γ-(4-vinylbenzyl)-l-glutamate N-carboxyanhydride (VB-l-Glu-NCA),20 γ-(4-allyloxylbenzyl)-lglutamate N-carboxyanhydride (AOB-l-Glu-NCA),21 and O-pentenyll- serine N-carboxylanhydride (PE-l-Ser-NCA)22) and photo-labile groups (e.g., γ-(4,5-dimethoxy-2-nitrobenzyl)-l-glutamate N-carboxylanhydride (DMNB-l-Glu-NCA)23] were synthesized and polymerized. The functional groups on the side chain of the resulting polyamino acids allow various chemical reactions to modulate the structures and properties of the materials. For instance, vinyl groups on poly-γ-(4-vinylbenzyl)-l-glutamate (PVBLG) side chains were converted to various functional groups including alcohol, aldehyde, carboxylic acid, vicinal diol, chloride, and aromatic ring via highly efficient post-polymerization modifications. The versatility of this chemistry shows great potential in generating varieties of polyamino acids with diverse side-chain functionalities and properties to broaden the applications of polyamino acid materials.
Water-soluble, Helical, Ionic Polyamino Acids
Helical conformation is one of the most common motifs of polyamino acids and is often associated with their biological activities. However, the usefulness of some natural α-helical polyamino acids (e.g., poly-l-leucine) toward biomedical applications is often limited by their poor aqueous solubility. On the other hand, natural polyamino acids with charged groups on their side chains have excellent water solubility but adopt random coil conformation due to the electrostatic repulsions between side-charged groups.24 Thus, substantial efforts have been directed toward the preparation of water-soluble polyamino acids with stable helical conformation. By introducing neutral hydrophilic groups (e.g., hydroxy group, sugar moieties, or oligo ethylene glycol) onto the side chains, polyamino acids with distinct helicity and aqueous solubility can be obtained.25-27 Unfortunately, these neutral polyamino acids are generally not useful for gene delivery due to their weak affinity for DNA.
To address this problem, we recently developed a class of ionic α-helical polyamino acids.28 When the side-chain ionic groups were placed distally from the polyamino acid backbone, the charge repulsion was minimized and the helical conformation was stabilized by the enhanced hydrophobic interaction due to the elongated hydrophobic side-chain (Figure 1). We discovered that when the charged residue was placed 11 σ-bonds away from the backbone, α-helical ionic polyamino acids with good water solubility were obtained (Figures 2A–2B). Using the conjugation strategy described above for PVBLG, a series of water-soluble polyamino acids (PVBLG-X) were prepared via ozonolysis and subsequent reductive amination of PVBLG (Scheme 1C). For all developed PVBLG-X, the positive-charged amino groups were 11 σ-bonds away from the backbone and showed up to 90% helicity. In addition, when the side chain charges were further situated 17 σ-bonds away from the backbone, polyamino acids with very high helicity (81%) can be obtained even at a low degree of polymerization (DP) of 10.21
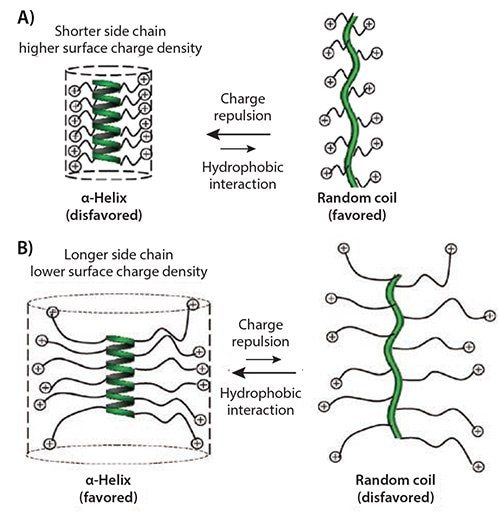
Figure 1. A) Illustration of polyamino acids with short, charged side chains and the postulated helix-to-random coil transition due to side-chain charge repulsion. B) Illustration of polyamino acids with long, charged side chains and postulated random coil-to-helix transition due to reduced side-chain charge repulsion and increased hydrophobic interaction.
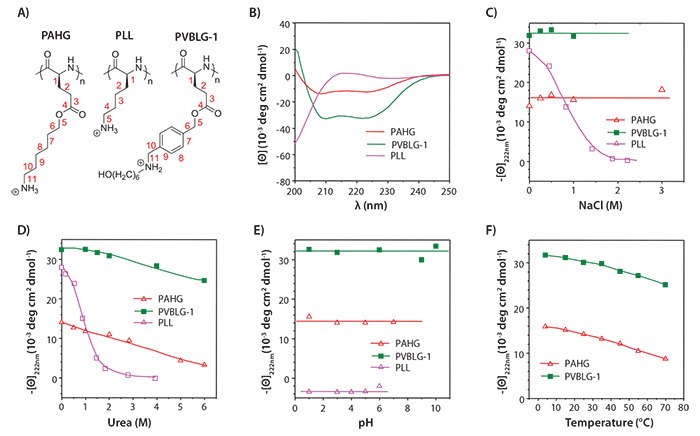
Figure 2. A) Chemical structure of PAHG, PLL, and PVBLG-1. B) Circular dichroism (CD) spectra of PAHG, PVBLG-1, and PLL in aqueous solution at pH 3. C) Salt concentration dependence of residue molar ellipticity at 222 nm for PAHG and PVBLG-1 at pH 3 and PLL at pH 10. D) The helical stabilities of PAHG and PVBLG-1 at pH 3 and PLL at pH 10 in the presence of urea. E) The pH dependence of the residue molar ellipticity at 222 nm for PAHG, PVBLG-1, and PLL. F) Temperature dependence of residue molar ellipticity at 222 nm for PAHG and PVBLG-1 at pH 3.
The helical stability of the α-helical ionic polyamino acids under various physiological conditions was evaluated with respect to biomedical applications. Particularly, unlike PLL, the helicity of PAHG and PVBLG-1 was stable against high ionic strength and in the presence of urea as the denaturing reagent (Figures 2C–2D). The helicity was also stable within a wide range of pH and temperature (Figures 2E–2F), both of which are important parameters for consideration in biomedical applications (such as the neutral pH of extracellular compartment and the acidic pH of the endosome). Such unique helical stability thus allows the polyamino acids to maintain their secondary structures and helicity-dependent membrane activities under a variety of complex biological conditions.
Role of Helicity in Cell Penetration and Gene Delivery
We also explored the potential of α-helical ionic polyamino acids toward non-viral gene delivery, wherein their sufficiently positively charged side chains enable effective DNA condensation, and the stable helical conformation potentially enables membrane transduction as noted in many CPPs.29 As part of our initial work, a library of cationic α-helical polyamino acids with different amine side groups was synthesized and screened in an attempt to identify particular candidates with proper balance between hydrophilicity (i.e., DNA binding strength) and hydrophobicity (i.e., membrane disruption potential). The top-performing material, PVBLG-8
(Figure 3A), showed stable helical conformation (Figure 3B) and yielded 12-fold higher transfection efficiency compared to the commonly used transfection reagent PEI (Figure 3C).
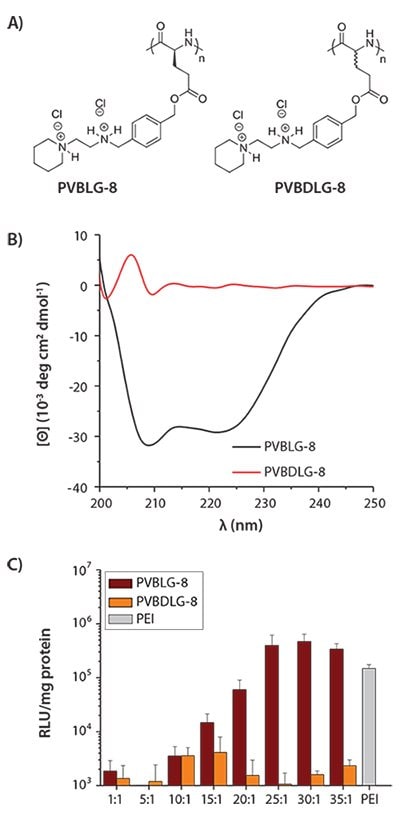
Figure 3. A) Chemical structure of helical PVBLG-8 and random-coiled PVBDLG-8. B) CD spectra of PVBLG-8 and PVBDLG-8 in aqueous solution at pH 7.4. C) In vitro transfection of PVBLG-8/pCMV-Luc complexes and PVBDLG-8/pCMV-Luc complexes in COS-7 cells at various molar ratios of amine to phosphate (N/P ratios). PEI (25 kDa) was included as a control.
Mechanistic studies were conducted to elucidate the function of the α-helical conformation in gene delivery. We showed that PVBLG-8 induced concentration-dependent destabilization of cellular or endosomal membranes (Figure 4A). To probe the endosomal escape mechanism, transfection studies were further performed in the presence of inhibitors such as bafilomycin that disrupts the proton sponge effect30 or nocodazole that leads to the accumulation of endocytosed material in early endosomes via the disruption of active cellular transport processes.31 Bafilomycin dramatically reduced the transfection efficiency of PEI, known as proton sponges, but had no effect on PVBLG-8 (Figure 4B). Comparatively, nocodazole induced a two-fold increase in the transfection efficiency of PVBLG-8, which indicated that the membrane disruption capabilities of PVBLG-8 increased with increasing polymer concentration (Figure 4B). These results collectively suggested that the incorporation of helical conformation, a trait shared by many CPPs, into our gene delivery vector yielded polyamino acids which possess the ability to destabilize endosomes by membrane disruption rather than the proton sponge effect. PVBDLG-8, a random-coiled racemic analogue of PVBLG-8 (Figures 3A–3B), was shown to have negligible transfection efficiency (Figure 3C), which highlights that polymer secondary structure can impact its overall performance. In order to test the breadth of applicability of this bioactive polyamino acid template, PVBLG-8 was also used to transfect cell lines that are traditionally considered to be hard to transfect, such as H9 human embryonic stem cells (hESC).32 The results revealed that, with the addition of nocodazole, PVBLG-8 exhibited a three-fold enhancement over the transfection efficiency of the commercially available transfection reagent, Lipofectamine® 2000 (LPF2000) (Figure 4C).
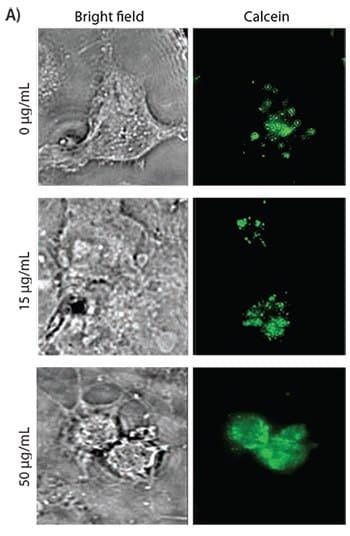
Figure 4A. Calcein uptake in COS-7 cells treated with various concentrations of PVBLG-8. Calcein is unable to cross intact membranes. As the concentration of PVBLG-8 in the extracellular medium is increased, the intracellular fluorescent signal becomes more diffuse, which indicates membrane permeation and non-endosomal uptake.
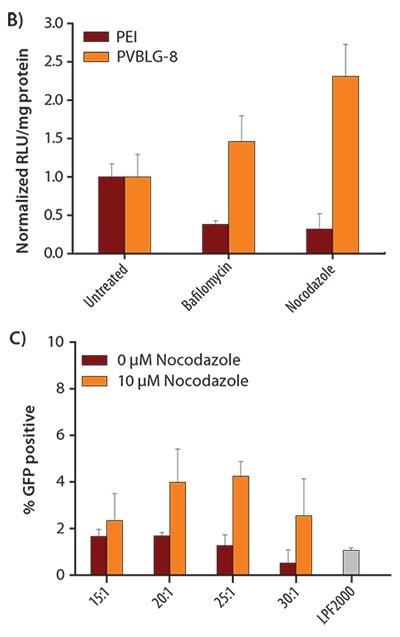
Figure 4. B) In vitro transfection of COS-7 cells treated with complexes of 25 kDa PEI or PVBLG-8 in the presence of intracellular inhibitors. The polymer concentration was fixed at 10 μg/mL. C) In vitro transfection of H9 human embryonic stem cells (hESC) with PVBLG-8 in the presence and absence of 10 μM nocodozole. Commercial transfection agent LPF2000 was included as a positive control.
Further Applications Based on PVBLG-8
In addition to DNA-based therapies, small interfering RNA (siRNA) has recently attracted much interest for the treatment of cancer and a number of other diseases. The RNA interference machinery features the site-specific mRNA cleavage and degradation in a highly efficient and specific manner, and thus enables gene regulation at the post-translation state. However, the successful clinical application of RNA interference has been hampered by the lack of efficient carriers or methods to deliver siRNA to target cells.33 CPPs have previously been utilized to mediate intracellular siRNA delivery34 and promote the endosomal escape of internalized siRNA-delivery vehicles. One major limitation of this approach, however, is that CPP activity depends on the formation of an acidic pH environment.35 Thus, if cargo is internalized via a process that avoids rapid acidification such as caveolae-mediated uptake (a pathway that has been identified as perhaps more efficient than traditional clathrin-mediated uptake), CPPs will be ineffective. To this end, the super-stable helical conformation of PVBLG-8 within a wide range of pH makes it an effective siRNA delivery vehicle which not only facilitates cellular internalization but also aids endosomal escape (Figures 5A–5B).36 Our results suggest that, unlike many other siRNA delivery vectors, PVBLG-8 operates by causing pore formation on cell membranes to allow direct diffusion of the siRNA cargo into the cell cytosol (Figure 5C). To further take advantage of such a unique property, PVBLG-8 was also incorporated into self-assembled nanoparticles in an attempt to mediate oral siRNA delivery.37 The stable helical structure of PVBLG-8 allows it to maintain helicity-dependent membrane permeabilities when passing through the acidic gastric tract and neutral intestinal lumen. The incorporation of PVBLG-8 could also alter the overall intracellular kinetics of complexes. For instance, upon the inclusion of PVBLG-8, supra-molecular self-assembled nano-complexes could enter the cells mainly through energy-independent permeation instead of conventional endocytosis.38
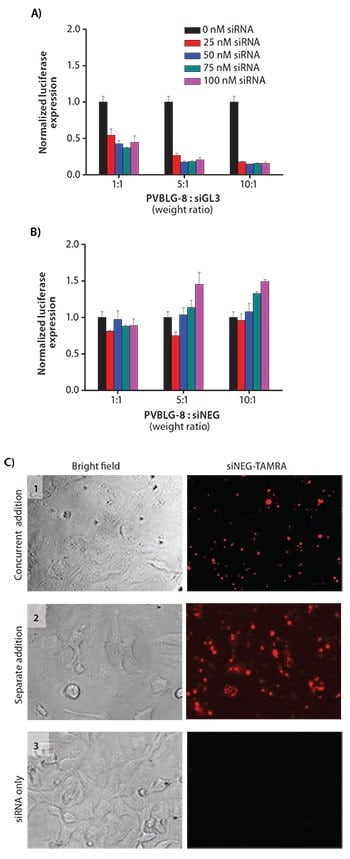
Figure 5. A) In vitro transfection of HeLa-Luc cells with luciferase siGL3 at various polymer:siRNA weight ratios and siRNA concentrations. B) In vitro transfection of HeLa-Luc cells with negative control siNEG. C) Fluorescence images of HeLa-Luc cells incubated with complexes formed with TAMRA-labeled siRNA and PVBLG-8. Free polymer and free siRNA were added sequentially in Pane 1. Free polymer was added, incubated, and removed prior to addition of free siRNA in Pane 2. Free siRNA was added without any polymer in Pane 3. The fluorescence signals observed in Panes 1 and 2 suggested that PVBLG-8 could cause pore formation on cell membranes to allow direct diffusion of siRNA into the cytoplasm.
Motivated by the desire to further understand the impact of polymer physicochemical properties (MW, charge density, threedimensional structure, chain flexibility, and hydrophilicity/ hydrophobicity balance, etc.) on the efficiency of non-viral gene vectors,39 we designed and synthesized various compositionally equivalent yet topologically different PEG-PVBLG-8 copolymers (Figure 6A). By using different initiators or grafting materials, di-block, ABA tri-block, graft, and star (8-arms) PEG-PVBLG-8 copolymers were obtained with desired molecular structures.40 Di-block and tri-block copolymers exhibited lower membrane activities and cytotoxicities due to the incorporation of PEG segments at the end of the polyamino acids. Nonetheless, these block copolymers showed uncompromised gene transfection efficiency compared to the non-PEGylated homopolymers. The graft copolymer, obtained by randomly grafting short PEG chains onto PVBLG-8, displayed lower DNA binding affinity and membrane activity, resulting in subsequently reduced transfection efficiency that is attributed to the steric effect induced by the PEG segments.
The star copolymer, initiated by an 8-arm PEG-NH2, demonstrated the highest membrane activity yet also relatively low cytotoxicity, thus resulting in a transfection efficiency that outperformed the non-PEGylated PVBLG-8 homopolymer and LPF2000 by 3–5 and 3–134 folds, respectively (Figures 6B–6C). The potency of the star copolymer was believed to result from its densely charged architecture as a “multivalent cationic sphere,” which substantially favored interactions with negatively charged cell membranes. Meanwhile, the lower cytotoxicity of the star copolymer as compared to the homopolymer is attributed to the reduced amount of individual PVBLG-8 moiety in direct contact with the cell membranes. Such studies on the structure–property relationship provide insight into the rational design of future synthetic gene vectors.
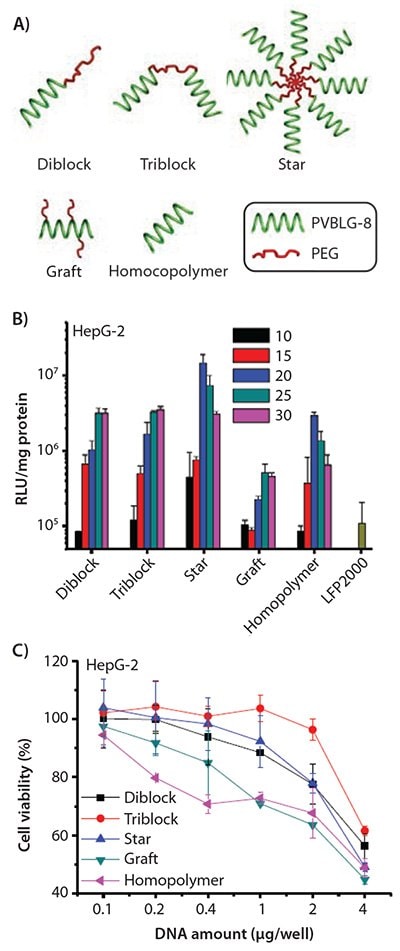
Figure 6. A) Schematic representation of PEG-PVBLG-8 copolymers with different architectures. B) Transfection efficiencies of polyplexes in HepG-2 cells at various molar ratios of amine to phosphate (N/P ratios). C) Cytotoxicity of polyplexes (N/P ratio of 20) toward HepG-2 cells as determined by the MTT assay.
Conclusions and Future Perspectives
We summarized our work on both the synthesis of watersoluble α-helical cationic polyamino acids and their biomedical applications in non-viral gene delivery. Using amine-TMS initiated NCA polymerization and post-modification of functional side chains, we were able to prepare a series of polyamino acid materials with different end-groups, architectures, and side-chain functionalities. In particular, by elongating the charged side chains to separate the charges from the polyamino acid backbone with a distance of 11 σ-bonds or longer, we were able to develop a series of cationic polyamino acids (PVBLG-X) with excellent water solubility and helical stability. Upon a screening process on PVBLG-X, PVBLG-8 was identified as the top-performing membranepenetrating and gene delivery material. Due to its secondary structure, PVBLG-8 facilitated cellular internalization and endosomal escape which improved its gene delivery efficiency either independently or in combination with other delivery systems. More specifically, our studies suggest that PVBLG-8 is able to penetrate cellular or endosomal membranes by creating pores on biological membranes, an energy-independent approach distinctly different from traditional endocytosis pathway.
In view of this reactive polyamino acid template, our current studies are focused on modifying the polyamino acids with trigger-responsive, degradable, targeting, or other functional moieties in order to further improve their efficacy for both in vitro and in vivo gene delivery. On the other hand, given the importance of secondary structure in biomedical applications and the ultrastability of helicity in this template, it presents great potential for therapeutics under extreme biological conditions, such as the very acidic environment of the gastric tract.
Acknowledgments
We acknowledge financial support from the NSF (CHE-1153122) and the NIH (NIH Director’s New Innovator Award 1DP2OD007246 and1R21EB013379).
References
如要继续阅读,请登录或创建帐户。
暂无帐户?