Centrifugation-free Protein Extraction and Purification from Total Cultures of Baculovirus-infected Insect Cells
The baculovirus expression vector system (BEVS) has been widely used for high-level expression of eukaryotic genes in insect cells.1,2 Cultured insect cells are capable of producing 100 mg target protein per 109 cells with baculovirus vectors expressing target genes under the control of AcNPV polh or p10 promoters.2 Insect cells readily carry out most of the post-translational modifications, such as glycosylation, phosphorylation, fatty acid acylation and myristorylation, that also occur in mammalian cells.34 These features make the BEVS attractive for the expression of mammalian proteins that are difficult to obtain in a soluble, active form in bacterial systems, or when post-translational modifications are required.
To express target genes using baculovirus vectors, growing cells are infected with recombinant viruses at a predetermined multiplicity of infection (M.O.I.) and harvested at an optimal time post-infection for recombinant protein purification. Because the polh and p10 promoters become fully active only very late during the lytic life cycle, optimal protein expression levels are often achieved when the culture has undergone partial lysis. As a result, at a typical time of harvesting approximately 25–30% of the cells have already undergone lysis, releasing precious recombinant proteins into the culture medium.5 This fraction is often discarded when the cells are collected by centrifugation prior to preparation of the cell extract.2,4 In addition, the steps required for cell harvest and preparation of extracts in buffers compatible with downstream affinity purification resins make processing multiple samples tedious, expensive and time-consuming.
Recently, extraction and purification of recombinant proteins expressed in bacteria have been greatly simplified by the use of specialized detergent-based reagents that eliminate the need for mechanical cell disruption.6,7,8 For example, the PopCulture™ Reagent enables efficient extraction of proteins from total bacterial cultures by completely eliminating centrifugation steps normally required for cell harvest and clearing lysates.7,8 Somewhat surprisingly, the yield and quality of His•Tag® and GST•Tag™ fusion proteins purified from 1-mL total culture extracts is generally superior to that obtained using conventional harvest and extract preparation methods.7, 8 The data are consistent with the conclusions that: 1) the total culture extraction method recovers target proteins from the culture medium that are normally lost during cell harvest, 2) proteins in the medium are not degraded under the conditions employed, and 3) the media and cellular components are compatible with affinity purification when using the PopCulture extraction protocol in combination with immobilized metal affinity chromatography (IMAC) or glutathione affinity chromatography.
The success of the PopCulture method with bacteria prompted us to investigate whether a similar approach could be developed for insect systems. Here we introduce a new detergent-based lysis reagent, Insect PopCulture™ Reagent, that is specially formulated for total culture extraction and affinity purification of recombinant proteins without the need for centrifugation of baculovirus-infected insect cells. The improved method increases processing efficiency and target protein yields, and is amenable for automated expression level screening and recombinant protein purification.
Purification of His•Tag® β-galactosidase from a total culture extract
Figure 1 depicts the overall procedure for direct in-media cell lysis methodology by using Insect PopCulture®. With this method, 0.05 volume of Insect PopCulture® Reagent is added to the culture, followed by 10 U/mL Benzonase® Nuclease to reduce viscosity arising from liberated nucleic acids. After a 15-minute incubation, the extract is ready for affinity purification; no clearing step is required. For testing purposes, we used a culture of TriEx™ Sf9 cells in serum-free TriEx Insect Cell Medium infected with a baculovirus recombinant expressing His•Tag® β-galactosidase fusion protein, and compared the Insect PopCulture® method with a conventional extraction and purification protocol that uses centrifugation to collect the cells and clear the lysate. The gel analysis in Figure 2 demonstrates that the Insect PopCulture® extraction method combined with Ni-NTA His•Bind® Resin purification produced a nearly homogeneous target protein that was indistinguishable from the protein purified using conventional extraction. The yield data also indicate that the total amount of target protein purified by the Insect PopCulture® method was approximately equal to the sum of the protein separately purified from the harvested cell pellet and supernatant fractions. The Insect PopCulture® method efficiently recovered target protein that had been released into the medium as well as the intracellular target protein. Although this experiment used a suspension culture, similar results were observed with adherent cells on tissue culture plates (data not shown).
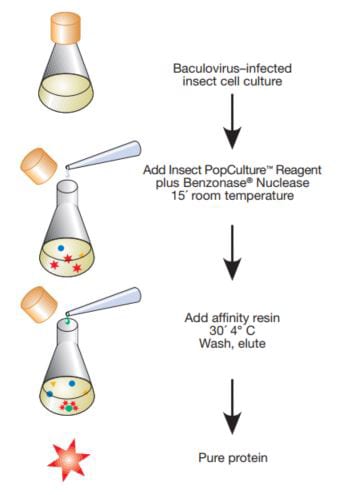
Figure 1.Insect PopCulture® Reagent method.
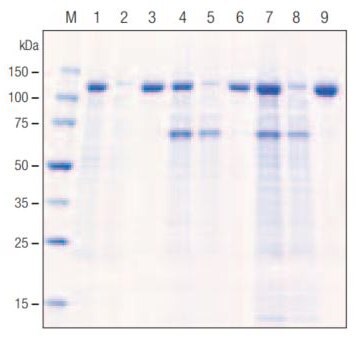
Figure 2.Purification of His•Tag® β-galactosidase from baculovirus-infected insect cell cultures.
| Lane | Sample |
|---|---|
| M | Perfect Protein™ Markers |
| 1 | Cell pellet, crude |
| 2 | Cell pellet, flow-through |
| 3 | Cell-pellet, eluate |
| 4 | Medium, crude |
| 5 | Medium, flow-through |
| 6 | Medium, eluate |
| 7 | Insect PopCulture™, crude |
| 8 | Insect PopCulture™, flow-through |
| 9 | Insect PopCulture™, eluate |
| Sample | Purified Protein |
|---|---|
| Cell pellet | 56 µg/mL culture |
| Medium | 64 µg/mL culture |
| Insect PopCulture | 131 µg/mL culture |
The bacterial β-galactosidase gene, lacZ, was PCR amplified and cloned into the pTriEx™-4 Ek/LIC Vector. Recombinant baculoviruses were generated by cotransfection using BacVector®-3000 Triple Cut Virus DNA according to the recommended protocol. For protein expression, TriEx™ Sf9 cells grown in a shaker culture in TriEx™ Insect Cell Medium were infected with baculoviruses at M.O.I. of 5. At 72 h post infection, 1 mL of the culture was used for direct in-media cell lysis with Insect PopCulture® Reagent, and 1 mL was processed by centrifugation, which produced cell pellet and media fractions. Insect PopCulture® reagent extraction was performed by the addition of 1/20th volume Insect PopCulture® Reagent, followed by 10 U/mL Benzonase® Nuclease. The mixture was gently inverted several times and incubated 15 min at room temperature. For the standard extraction, the cell pellet was resuspended in an equal culture volume (1 mL) CytoBuster™ Protein Extraction Reagent. After 15 min incubation at room temperature, cell debris was removed by centrifugation. The media fraction was treated with Insect PopCulture® reagent in parallel with the total culture sample. For affinity purification, the extracts were used to resuspend 0.2 mL equilibrated Ni-NTA His•Bind® Resin and incubated 30 min at 4 °C on an end-over-end shaker. The slurries were poured into disposable 5 mL columns. Columns were washed with 20 bed volumes of 1X Ni-NTA Wash Buffer. The His•Tag® β-galactosidase fusion protein was eluted with 1X Ni-NTA Elute Buffer in 0.5-mL fractions. Protein concentration of the pooled eluates was determined by the BCA assay. The crude extract, flow through, and pooled eluates were analyzed by SDS-PAGE and Coomassie blue staining. Lanes are indicated.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?