Performing a Purification of NADP+-dependent dehydrogenases and other enzymes with affinity for NADP+ Using 2’5’ ADP Sepharose® 4B and Red Sepharose® CL-6B
NADP+-dependent dehydrogenases interact strongly with 2’5’ ADP. Selective elution with gradients of NAD+ or NADP+ has allowed the resolution of complex mixtures of dehydrogenase isoenzymes using 2’5’ ADP Sepharose® 4B.
Synthesis of the medium takes place in several steps. Diaminohexane is linked to 2’5’ ADP via the N6 of the purine ring. The derivatized ADP is then coupled to Sepharose® 4B via the aminohexane spacer. Figure 44 shows the partial structure of 2’5’ ADP Sepharose® 4B.
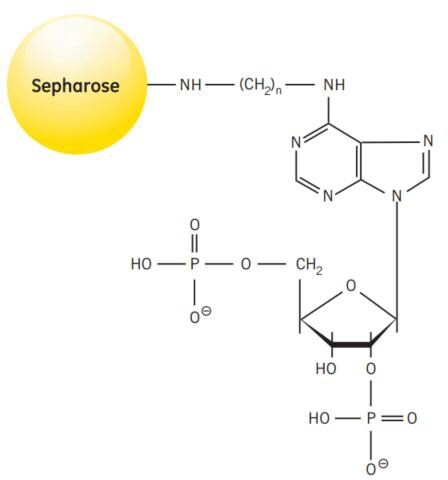
Figure 44.Partial structure of 2’5’ ADP Sepharose® 4B.
NADP+-dependent dehydrogenases are also members of a larger group of proteins that will interact with Procion™ Red, a synthetic polycyclic dye that shows certain structural similarities to naturally occurring NADP+. When used as an affinity ligand attached to Sepharose® CL-6B, Procion Red HE-3B will bind strongly and specifically to a wide range of proteins. Some proteins bind specifically due to their requirement for nucleotide cofactors, while others, such as albumin, lipoproteins, blood coagulation factors and interferon, bind in a less specific manner by electrostatic and/or hydrophobic interactions with the aromatic anionic ligand.
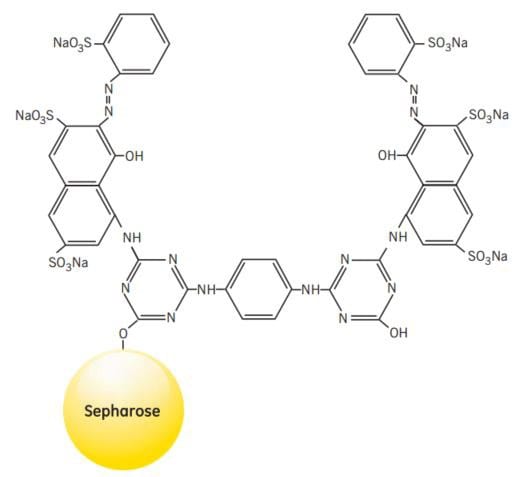
Figure 45.Partial structure of Red Sepharose® CL-6B
2’5’ ADP Sepharose® 4B
Purification Example
Figure 46 shows a linear gradient elution used for the initial separation of NADP+-dependent enzymes from a crude extract of Candida utilis.
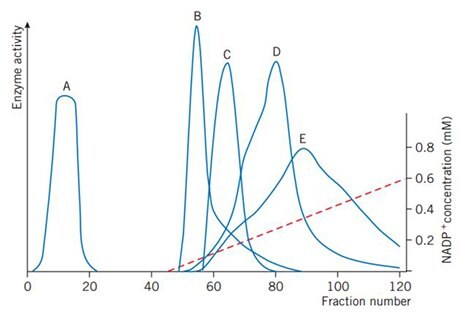
Figure 46.Gradient elution with 0–0.6 mM NADP+. A: non-interacting protein, B: glucose-6-phosphate dehydrogenase, C: glutamate dehydrogenase, D: glutathione reductase, E: 6-phosphogluconate dehydrogenase. (Brodelius et al., Eur. J. Biochem. 47, 81–89 (1974)).
Performing a Separation
Swell the required amount of powder for 15 min. in 0.1 M phosphate buffer, pH 7.3 (100 ml per gram dry powder) and wash on a sintered glass filter (porosity G3). Pack the column (see Appendix 3, Column packing and preparation).
Binding buffer: 10 mM phosphate, 0.15 M NaCl, pH 7.3
If the protein of interest binds to the medium via ionic forces, it may be necessary to reduce the concentration of NaCl in the binding buffer.
Elution buffers: use low concentrations of the free cofactor, NAD+ or NADP+ (up to 20 mM) with step or gradient elution.
If detergent or denaturing agents have been used during purification, these can also be used in the low and high pH wash buffers.
Cleaning
Wash 3 times with 2–3 column volumes of buffers, alternating between high pH (0.1 M Tris-HCl, 0.5 M NaCl, pH 8.5) and low pH (0.1 M sodium acetate, 0.5 M NaCl, pH 4.5). Re-equilibrate immediately with 3–5 column volumes of binding buffer.
Remove denatured proteins or lipids by washing the column with 2 column volumes of detergent e.g. 0.1% Triton X-100 for 1 minute. Re-equilibrate immediately with 5 column volumes of binding buffer.
Chemical Stability
Stable to all commonly used aqueous buffers and additives such as detergents. Avoid high concentrations of EDTA, urea, guanidine hydrochloride, chaotropic salts and strong oxidizing agents. Exposure to pH > 10 may cause loss of phosphate groups.
Storage
Store freeze-dried product below +8 °C under dry conditions.
Wash media and columns with 20% ethanol at neutral pH (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
Red Sepharose® CL-6B
Performing a separation
Swell the required amount of powder for 15 min. and wash with distilled water on a sintered glass filter (porosity G3). Use 200 ml water for each gram of dry powder, adding in several aliquots. One gram of freeze-dried material gives a final volume of approximately 4 ml. Pack a column (see Appendix 3, Column packing and preparation).
Binding buffer: Use a buffer at around neutral pH since proteins bind specifically to Red Sepharose® CL-6B at this pH.
The binding capacity will depend upon parameters such as sample concentration, flow rate, pH, buffer composition and temperature. To obtain optimal purification with respect to capacity, determine the binding capacity over a range of different pH and flow rates.
Elution buffers: use low concentrations of the free cofactor, NAD+ or NADP+ (up to 20 mM), or increase ionic strength up to 2 M NaCl or 1 M KCl.
If detergent or denaturing agents have been used during purification, these can also be used in the low and high pH wash buffers.
Cleaning
Wash 3 times with 2–3 column volumes of buffers, alternating between high pH (0.1 M Tris-HCl, 0.5 M NaCl, pH 8.5) and low pH (0.1 M sodium acetate, 0.5 M NaCl, pH 4.5). Re-equilibrate immediately with 3–5 column volumes of binding buffer.
Remove denatured proteins or lipids by washing with 2 column volumes of 6 M guanidine hydrochloride or 8 M urea. Alternatively, wash the column with 2 column volumes of detergent in a basic or acidic solution, e.g. 0.1% Triton X-100 in 1 M acetic acid. Remove residual detergent by washing with 5 column volumes of 70% ethanol. In both cases wash immediately with 5 column volumes of binding buffer.
Chemical Stability
Stable with all commonly used aqueous buffers and additives such as 8 M urea and 6 M guanidine hydrochloride.
Storage
Store freeze-dried powders under dry conditions and below +8 °C.
Wash media and columns with 20% ethanol in 0.1 M KH2PO4, pH 8.0 (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?