阿尔茨海默病生物标记物多重和高灵敏度免疫测定
对于阿尔茨海默病等复杂神经退行性疾病,可结合多重和高灵敏度免疫测定技术检测其生物标志物,帮助研究人员更深入地了解疾病机制。本文介绍了如何结合MILLIPLEX®多重和SMC®高灵敏度免疫测定技术,检测人脑脊液、血浆和血清样本中阿尔茨海默病(AD)生物标志物。
AD研究为何要结合使用多重和高灵敏度免疫测定技术?
监测阿尔茨海默病(AD)患者的脑脊液(CSF)中的蛋白质生物标记物,对于了解疾病的进展极具价值1。多重免疫测定可让研究人员通过一次实验同时测定多种生物标志物,获得大量可靠数据。尽管几种CSF生物标记物能够可重现地区分正常和患病样品,但CSF是一种很难在研究中获得的生物液。
对基于血液的AD生物标志物的需求推动了对新型候选蛋白的不懈探索2。然而,血液来源生物标志物的发现可能受标准免疫测定灵敏度限制。高灵敏度免疫测定技术可检测各种生物液中的低丰度蛋白,为发现新型生物标记物创造了机会,彻底革新了神经退行性疾病研究3,4。
如何结合使用多重和高灵敏度免疫测定技术
为证明单分子计数(SMC®)技术对各种样本中的阿尔茨海默病生物标志物的超灵敏定量能力,使用来自AD患者和健康对照的CSF、血浆和血清(先)对MILLIPLEX®神经科学相关试剂盒进行评估。
人CSF、血浆和血清由商业供应商(Discovery Life Sciences、BioIVT和PrecisionMed)取自AD患者和无AD正常对照。按照试剂盒的实验方案要求,不稀释或稀释样品。通过非配对t检验(unpaired t-test)计算所有分析物的P双尾值(two-tail p-value)(GraphPad Prism软件)。
按照产品说明书要求,在96孔板中进行MILLIPLEX®多重测定。在Luminex® 200™系统上测定平均荧光强度(MFI),并用MILLIPLEX® Analyst 5.1软件分析数据。
采用的多重测定试剂盒如下:
- 采用MILLIPLEX®人β-淀粉样蛋白和Tau蛋白Panel(货号 HNABTMAG-68K)定量以下分析物:1:2稀释CSF样本中的β-淀粉样蛋白(1-40)(Aβ40)、β-淀粉样蛋白(1-42)(Aβ42)、总Tau蛋白(tTau)和pTau T181。
- 采用MILLIPLEX®人神经科学Panel 1(货号 HNS1MAG-95K)定量以下分析物:CSF未稀释样本中的α-突触核蛋白、胶质纤维酸性蛋白(GFAP)、神经元特异性烯醇化酶(NSE)、PARK5 (UCHL1)、PARK7 (DJ-1)和转谷氨酰胺酶2(TGM2)。
- 采用MILLIPLEX®人神经科学Panel 2(货号 HNS2MAG-95K)定量以下分析物:1:10稀释CSF、血清和血浆样品中的血管生成素(ANG)、ApoE4、FABP3、铁蛋白、神经颗粒蛋白(NGRN)和TREM2。该panel已通过CSF和血液(血清和血浆)样品检测验证。
采用SMC®β-淀粉样蛋白1-40高灵敏度免疫测定剂盒(货号 03-0145-00)和SMC®-淀粉样蛋白1-42免疫测定试剂盒(货号 03-0146-00),根据产品说明书定量分析β-淀粉样多肽。分别采用ERENNA®和SMCxPRO®仪器两款仪器,定量分析SMC®免疫测定结果:。ERENNA®和SMCxPRO®平台分别采用Sgx Link™和xPRO®软件进行数据采集和分析。
多重测定结果
MILLIPLEX®多重免疫测定是公认的神经退行性疾病生物标志物测定重要工具。
CSF样品
先测定正常和AD患者CSF样品中的几种经典和新型神经退行性疾病生物标记物,再测定血清和血浆样品中的生物标记物。测定所用的各试剂盒的标准曲线如图1所示。

图 1.利用多重免疫测定技术进行神经科学研究。MILLIPLEX® (A)人β-淀粉样蛋白和Tau蛋白试剂盒、(B)人神经科学Panel 1和(C)人神经科学Panel 2的标准曲线。
采用多因子试剂盒检测AD患者和健康对照(正常)的CSF样品,测定两者在特定生物标记物上的浓度差异。每种试剂盒都反映了AD CSF样品相比对照样品的改变(图2)。

图 2.正常和AD CSF样品的多重生物标记物分析。
采用MILLIPLEX®神经科学试剂盒测定人CSF中的16种蛋白。每种试剂盒都分析了正常和AD CSF两种样品,揭示两者的生物标志物差异:磷酸化Tau(p=0.0009)、Aβ42(p=0.0476)、NSE(p=0.008)、GFAP(p=0.0016)、UCHL1(p=0.0088,数据未显示)和神经颗粒蛋白(p=0.0359)。
每种试剂盒的样品量如下:
- 人β-淀粉样蛋白和Tau蛋白Panel:正常CSF,n=14;AD CSF,n=16
- 人神经科学Panel 1:正常CSF,n=15;AD CSF,n=8
- 人神经科学Panel 2:正常CSF,n=7;AD CSF,n=7
在人β-淀粉样蛋白和Tau蛋白Panel检测中,AD CSF的Aβ42下降,磷酸化-Tau(Thr181)升高,这些经典AD生物标记物的分析结果与已有的结果一致5。根据人神经科学Panel 1的测定,AD CSF中的NSE和GFAP浓度均有上升。在人神经科学Panel 2的分析中,AD CSF的神经颗粒蛋白NRGN浓度高于对照。
血清和血浆样本
人神经科学Panel 2还可用于检验血清和血浆样本,于是我们接着检查了循环系统中的分析物是否具有蛋白表达差异。AD患者血浆和血清的FABP3均升高,AD血清的TREM2升高,AD血浆的NRGN升高(图3)。
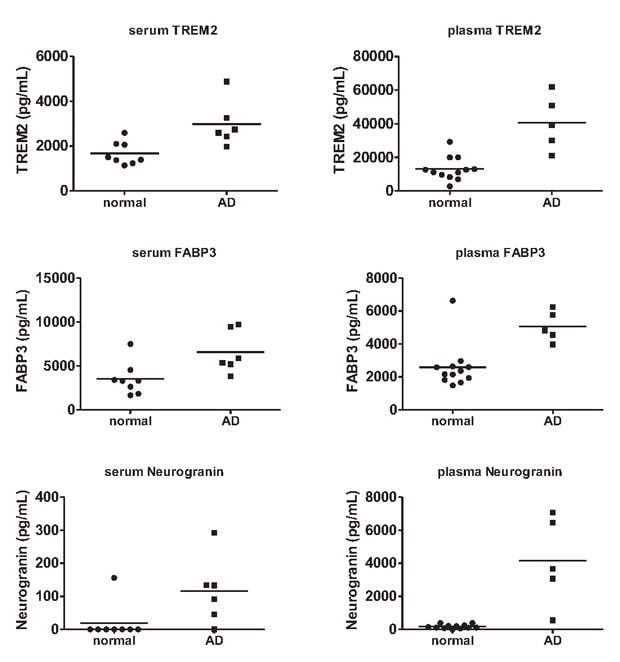
图 3.正常和AD血浆和血清样品的多重AD生物标记物测定。
使用MILLIPLEX®人神经科学Panel 2,测定AD患者血浆(n=5)和血清(n=6)与健康对照血浆(n=12)和血清(n=8)中的疾病相关蛋白。通过人神经科学Panel 2对血清和血浆的分析发现,AD样品相比健康对照,TREM2(血清p=0.0083)、FABP3(血清p=0.0204,血浆p=0.002)和神经颗粒蛋白(血浆p=0.0044)升高。
高灵敏度测定结果
尽管(通过上述多重测定结果发现)多种分析物具有成为基于血液的AD生物标志物潜力,但其他血液低丰度蛋白的分析必须依靠SMC®技术的高灵敏度测定平台。SMC®技术可测定人血浆样品中的β-淀粉样多肽(图4)。AD血浆Aβ40(p=0.028)略微升高。血浆Aβ42未见变化。重要的是,SMC® Aβ42试剂盒成功测定到CSF的Aβ42 (p=0.0116)下降,这属于AD的特征性表现。CSF的Aβ40(p=0.0055)水平也有变化。Aβ40和Aβ42试剂盒均相继在SMCxPRO®器、ERENNA®仪器上进行读数,显示SMC®的不同检测平台具有出色的相关性。
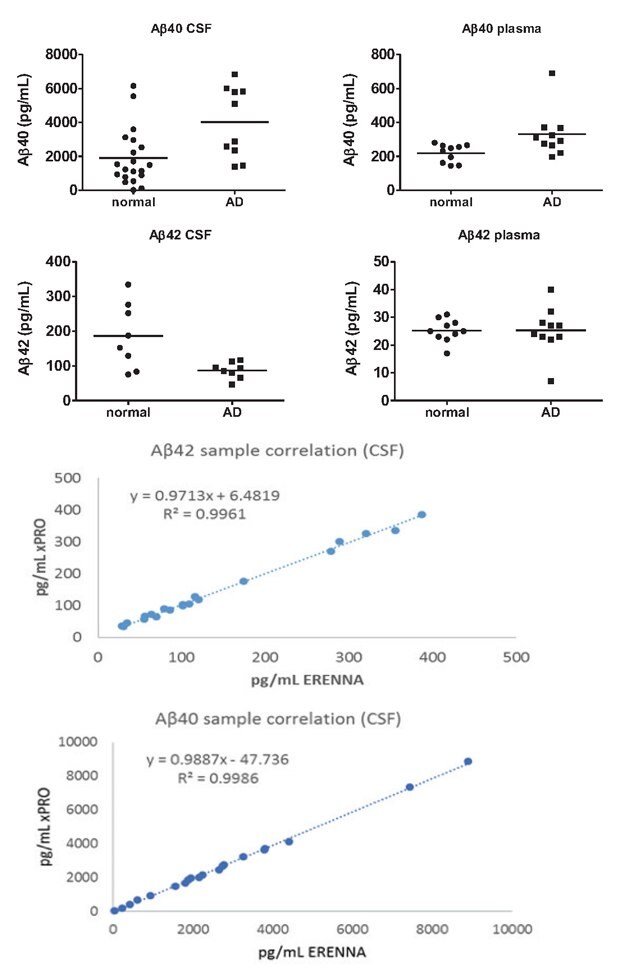
图 4.高灵敏度免疫测定正常和AD血浆、血清、CSF样品生物标记物时,不同检测仪器的相关性。
分别使用相应的SMC®高灵敏度免疫测定试剂盒,测定AD患者和正常对照CSF和血浆中的Aβ40和Aβ42水平。AD患者和健康对照的血浆(p=0.028)和CSF(p=0.0055)Aβ40均观测到差异。AD CSF(p=0.0116)中的Aβ42下降,血浆(p=0.9733)中未见变化。先后在SMCxPRO®仪器和ERENNA®仪器上分析测定CSF的同一反应板,结果证明这两种平台具有高度相关性,如图4所示。
每种试剂盒的样品量如下:
- SMC®β-淀粉样蛋白1-40高灵敏度免疫测定试剂盒:正常CSF(n=20),AD CSF(n=10),正常血浆(n=10),AD血浆(n=10)
- SMC®β-淀粉样蛋白1-42高灵敏度免疫测定试剂盒:正常CSF(n=8),AD CSF(n=8);正常血浆(n=10),AD血浆(n=10)
材料表
参考文献
如要继续阅读,请登录或创建帐户。
暂无帐户?