β-Alanine in Cell Culture
What is β-Alanine?
β-alanine, or 3-aminopropanoic acid, is an amino acid in which the amino group is attached to the second carbon atom away from the carboxylate group, also known as the β-carbon. β-alanine is the only naturally occurring β-amino acid found in animals, plants, and microorganisms. Unlike its isomer α-alanine, β-alanine does not have a stereocenter, so it has no stereoisomers.
Figure 1.Structure of A) β-alanine; B) L-α-alanine.
β-alanine was identified as a constituent amino acid of carnosine by Russian scientist Gulewitsch in the early 1900s1 . It is classified as a non-essential amino acid and is produced by degradation of dihydrouracil and carnosine in vivo.
Related Products
β-alanine is not encoded in the genome of organisms for protein assembly. Although it is not incorporated into proteins, β-alanine is a component of many important naturally occurring products including dipeptide carnosine, anserine, and pantothenic acid, also known as vitamin B5, a component of coenzyme A.
β-alanine is the rate-limiting precursor of carnosine, a dipeptide that has antioxidant properties and is found in high concentrations in skeletal muscle, gastrointestinal tissues, and the human brain. Carnosine is synthesized from β-alanine and L-histidine through carnosine synthase (EC 6.3.2.11) in the presence of ATP2 . The reaction can be reversed, with the dipeptide degraded by carnosinase (EC 3.4.13.3) to β-alanine and L-histidine.
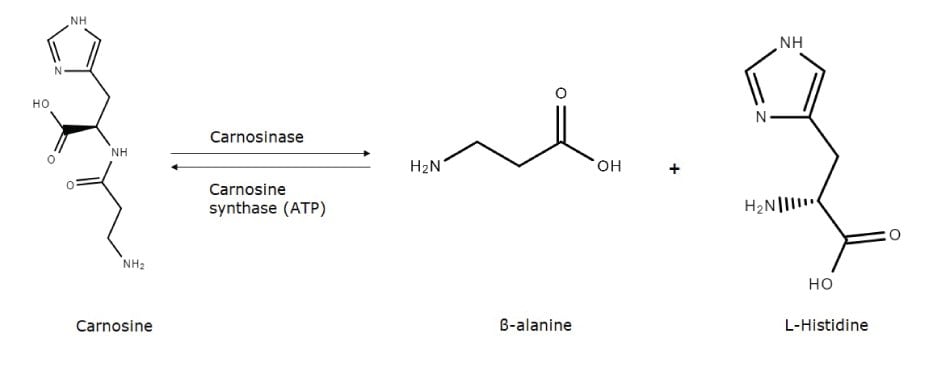
Figure 2.The metabolic pathway between carnosine and its constituent amino acids β-alanine and L-histidine.
β-Alanine Function in Cells
β-alanine has important physiological functions in the metabolism of animals, plants, and microorganisms. One of the main functions of β-alanine in cells is to serve as a precursor for the synthesis of carnosine, a molecule that helps regulate pH levels in cells.
Carnosine acts as a buffer, neutralizing the accumulation of hydrogen ions that can lead to acidification of the cell. β-alanine is taken up by cells and used to synthesize carnosine, improving cell growth and viability.
β-alanine also acts as a precursor to anserine, another dipeptide synthesized in the cell. Both carnosine and anserine have been shown to have antioxidant and anti-inflammatory properties and may contribute to maintaining cellular health and function. Thus, β-alanine flux plays a cytoprotective role to the cells exposed to hypoxic stress.
β-alanine is also a small molecule neurotransmitter. It is a nonselective agonist at glycine receptors and a ligand for the G protein-coupled orphan receptor, TGR7 (MrgD) in neurons3 . β-alanine is released from the neurons when they respond to electrical stimulation and inhibits neuronal excitation4 .
In E. coli, β-alanine is also a direct precursor in the biosynthesis of pantothenic acid, or vitamin B5, and is crucial for bio-technological vitamin B5 production.
β-Alanine in Cell Culture
Because it can increase the production of carnosine, which helps regulate pH levels in cells, β-alanine is often used as a cell culture supplement. Because carnosine helps neutralize hydrogen ions in the culture media, β-alanine supplementation can help prevent acidification in culture media and improve cell growth and viability.
Not all cell lines require β-alanine in their growth medium. β-alanine supplementation depends on various factors such as the type of cell line, its metabolic demands, the pH of the growth medium, and the duration of the cell culture. Some cell lines, such as skeletal, cardiac, and smooth muscle cells, have a higher demand for carnosine and therefore may benefit from β-alanine to maintain a proper pH5[NK1] . Neuronal cells and some cancer cell lines may also benefit from β-alanine supplementation.
β-alanine may not be necessary and can even have adverse effects for some other cells. For example, non-malignant MCF-10a and malignant MCF-7 breast epithelial cells showed suppressed cell migration and proliferation by the addition of β-alanine6 .
Chemical Characteristics Of β-Alanine
β-alanine has the molecular formula C3H7NO2 and molecular weight of 89.093 g/mol. It has an isoelectric point of 6.9 and pKa of 3.55 and 10.24.
β-alanine is relative stable in powder form. It should be stored in a well-sealed container and placed in cool, dry, and dark place. Exposure to high temperature, moisture, or light will cause degradation of β-alanine and reduce its effectiveness.
References
To continue reading please sign in or create an account.
Don't Have An Account?