γ-Valerolactone (GVL): A Bio-Based Dipolar Aprotic Solvent for More Sustainable Synthesis
Abstract
The advancement of more sustainable practices in organic synthesis increasingly hinges on the adoption of bio-based solvents. Traditional dipolar aprotic solvents such as N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), and N,N-dimethylacetamide (DMAc) are widely used but present serious environmental and safety concerns due to their toxicity, poor biodegradability, and regulatory restrictions. γ-Valerolactone (GVL, 933856), a bio-based solvent derived from lignocellulosic biomass, offers synthetic chemists a compelling alternative solvent with favorable properties including high polarity, thermal stability, low toxicity, and excellent biodegradability. This application note demonstrates the potential of GVL to replace conventional solvents in key organic transformations—including Pd-cross coupling, nucleophilic aliphatic and aromatic substitution, and amidation—while maintaining comparable or improved reaction efficiency. By highlighting GVL’s broad applicability and environmental advantages, this work positions it as a practical, more sustainable alternative for modern organic synthesis.
Section Overview
Introduction
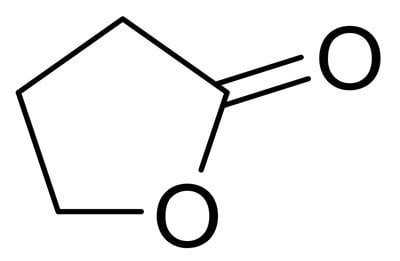
Figure 1.Structure of γ-Valerolactone (GVL).
Conventional solvents such as N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), N,N-dimethylacetamide (DMAc), 1,4-dioxane and tetrahydrofuran (THF) offer strong solvating capabilities but are associated with toxicity, regulatory pressure, and poor biodegradability1a-i. The chemical industry is therefore actively seeking high-performance, bio-based alternatives to reduce their environmental footprint. γ-Valerolactone (GVL, Figure 1) is one such candidate, derived from lignocellulosic biomass via the catalytic hydrogenation and cyclization of levulinic acid.1k-l
As a biomass-derived, biodegradable (OECD Test Guideline 301B) and low-toxicity solvent, GVL conforms to multiple principles of “Green Chemistry”1j, notably Principle 5 (safer solvents and auxiliaries), Principle 7 (use of renewable feedstocks) Principle 10 (design for degradation) and Principle 3 (less hazardous chemical syntheses), supporting its adoption as a more sustainable alternative in synthetic and formulation processes. Its properties – namely high polarity, thermal stability, low vapor pressure, and miscibility with both aqueous and organic media (Tables 1 & 2) - make it a versatile replacement for hazardous dipolar aprotic solvents in many synthetic applications.
GVL has been highlighted in solvent selection frameworks such as the GSK solvent sustainability guide1c and the CHEM21 solvent selection guide1d, which systematically rank solvents based on safety, health, and environmental metrics. Both resources recommend GVL due to its low flammability, low toxicity, and excellent environmental profile. As such, GVL is being increasingly adopted in key transformations including Pd-catalyzed cross-coupling, nucleophilic aromatic substitution and amide bond formation, where it has shown yields comparable to or better than conventional solvents.2
This application note focuses on evaluating GVL’s performance in key reaction classes that are commonly dependent on dipolar aprotic solvents: nucleophilic substitution, amidation and palladium-catalyzed cross-coupling. These reactions were selected because they are foundational to pharmaceutical and fine chemical synthesis and typically require solvents with strong polar characteristics. By demonstrating GVL’s effectiveness in these transformations, we aim to provide practical guidance for its adoption in greener synthetic workflows.
GVL as a Greener Alternative to Hazardous Organic Solvents
GVL offers a compelling combination of high performance and sustainability characteristics, making it an appealing more sustainable alternative to traditional dipolar aprotic solvents. Derived from renewable resources, it addresses increasing regulatory and safety pressures by delivering comparable reactivity without the associated toxicity or persistence3. Its compatibility with diverse reaction conditions - ranging from cross-couplings to nucleophilic substitutions - further supports its role in greener synthetic workflows. As such, GVL stands out as a practical and forward-looking solution for modern organic synthesis. Here are few application examples for GVL to replace hazardous solvents in industry.
Pharmaceutical and Polymer Manufacturing
GVL’s solvating power enables it to substitute effectively for hazardous solvents in pharmaceutical synthesis, polymer processing, and crystallization steps, offering comparable performance with enhanced safety. Fodor4a et al. (2020) demonstrated GVL’s successful application in homogeneous, phosphine-free Pd-catalyzed Heck coupling reactions. Their study revealed high functional group tolerance, moisture stability, and catalytic performance comparable to DMF. Detailed kinetic and computational analyses confirmed that GVL supports efficient catalysis under mild conditions without altering the reaction mechanism. Complementing this, Kerkel4b et al. (2021) provided a comprehensive environmental and physicochemical profile of GVL, confirming its ready biodegradability and low toxicity across various aquatic species. Moreover, modeling approaches such as Hansen solubility parameters and COSMO-RS indicated that GVL closely resembles solvents like DMF and NMP, underscoring its potential as a safer substitute in pharmaceutical, polymer, cleaning, and cosmetic applications.
Further expanding on GVL’s catalytic utility, Strappaveccia4c et al. applied it in Pd-catalyzed Heck–Mizoroki coupling reactions for both small-molecule synthesis and the production of conjugated polymers such as poly(phenylenevinylene) (PPV) derivatives. GVL demonstrated comparable polarity, high thermal stability, and low toxicity while significantly reducing palladium leaching - achieving residual metal contents as low as 6 ppm compared to over 800 ppm in NMP. This reduction minimizes downstream purification requirements and enhances the overall sustainability of the process. The resulting polymeric materials exhibited excellent optoelectronic performance in organic photovoltaic and transistor devices, validating GVL’s suitability for greener and scalable synthesis of semiconducting materials.
Together, these studies establish GVL as a more sustainable, low-toxicity, and high-performance solvent with broad applicability in greener chemistry, particularly in pharmaceutical manufacturing, catalysis, and advanced materials processing.
Printed Electronics
GVL has also emerged as a transformative greener solvent in the field of printed electronics, particularly in perovskite solar cell (PSC) fabrication. Derived from renewable biomass, GVL offers a low-toxic, biodegradable alternative to conventional solvents like DMF and γ-butyrolactone (GBL). While GBL is not highly toxic, its psychoactive properties and associated legal restrictions pose significant barriers to widespread adoption. The 2021 study by Worsley5a et al. demonstrated that GVL can effectively replace GBL in mesoscopic carbon-based PSCs, achieving comparable power conversion efficiencies (PCEs) and enhanced device stability under prolonged illumination and thermal stress. Complementing this, Miao5b et al. (2023) showcased GVL’s exceptional ink stability—maintaining performance after one year of storage—and its compatibility with scalable processing, enabling the fabrication of high-efficiency PSCs (PCE >25%) and certified mini-modules (>20%) without the need for toxic antisolvents. Together, these studies establish GVL not only acts as a safer and more sustainable solvent but also as a performance-enhancing material for next-generation photovoltaic technologies.
Co-solvent with Ionic Liquids
GVL emerges as a greener, more sustainable, and highly effective solvent for polymer dissolution, offering a low-toxic alternative to conventional harsh chemicals. In combination with imidazolium-based ionic liquids like [C₈MIm][Cl], GVL exhibits remarkable synergistic effects, significantly enhancing polymer solubility. This innovative solvent system not only enables efficient processing of robust polyaramids but also aligns with principles of Green Chemistry, paving the way for more sustainable materials science and extraction technologies.6
Results and Discussion
This application note presents a series of representative organic reactions to demonstrate the versatility and effectiveness of GVL as a renewable dipolar aprotic solvent. These examples highlight its ability to replace conventional polar aprotic solvents such as DMF, NMP, and DMAc in common chemical transformations without compromising efficiency or selectivity. A summary of the reaction conditions and yields is provided in Table 3.
The selected reactions include amide bond formation, nucleophilic substitution, and palladium-catalyzed C–C coupling, all of which are relevant to pharmaceutical and fine chemical synthesis. These reactions are commonly carried out in DMF, NMP and DMAc due to their strong solubilizing ability and polarity. GVL’s comparable solvating power, high thermal and chemical stability, and low toxicity make it a promising alternative across these reaction classes.
Key examples include acid–amine coupling using HATU and DIPEA in GVL, enabling high-yielding amide formation under mild conditions. GVL demonstrated good performance as a reaction medium in the synthesis of peptidomimetics, amino acid derivatives, and functionalized small molecules, achieving yields in the range of 75–85% in multiple cases. In addition, GVL was successfully used in a Pd(II)-catalyzed cross-coupling reaction involving tert-butyl (4-bromobenzyl)carbamate and 4-methylthiazole, showing excellent compatibility with organometallic catalysis and replacing NMP effectively.
GVL also supported more challenging transformations like the synthesis of substituted isoindolinones and diazaspiro compounds, both of which benefit from the solvent’s ability to dissolve polar intermediates and facilitate clean product isolation via precipitation or extraction. In reactions requiring column purification, GVL left minimal residue and showed easy removability under reduced pressure.
These diverse examples reinforce GVL’s practical utility as a viable substitute for traditional dipolar aprotic solvents in both batch and potential continuous-flow applications. The reaction protocols employed standard laboratory conditions and illustrated GVL’s compatibility with scalable, more sustainable synthetic chemistry. Details of all experimental conditions and spectral data are provided in the Appendix.
Substitution Reactions
Substitution reactions are widely used in synthetic chemistry and represent a practical context to evaluate greener solvent alternatives. This application note investigates the performance of GVL, a more sustainable solvent, in facilitating representative substitution reactions - including halogenation and O-/N-alkylation - commonly employed in synthetic laboratories. Our experimental results demonstrate that GVL can successfully replace conventional dipolar aprotic solvents such as THF, 1,4-dioxane, NMP, DMF, and dimethylacetamide (DMAc), with no loss in yield or efficiency. These findings support GVL’s utility as a viable and more sustainable solvent platform for both academic and industrial synthesis.
Nucleophilic Aromatic Substitution (SNAr) Reaction
Nucleophilic aromatic substitution (SNAr) is a key strategy for functionalizing electron-deficient aromatic systems, proceeding via a Meisenheimer complex. Solvent choice critically influences reaction efficiency, with polar aprotic solvents like DMF, NMP, DMAc, THF and 1,4-dioxane commonly used to stabilize intermediates and enhance nucleophilicity. Recent studies highlight solvent-dependent mechanistic variations, including shifts toward single-electron transfer (SET) pathways. In this context, GVL emerges as a greener alternative, effectively supporting SNAr reactivity.7a,b In the example below (Figure 2), 2-(2,6-dioxopiperidin-3-yl)-4-fluoroisoindoline-1,3-dione (1) was reacted with 1-Boc-piperazine (343536) in GVL under heating conditions to afford tert-butyl 4-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)piperazine-1-carboxylate (2) in 87% yield, whereas the same reaction yields are 82% and 79% in DMF and NMP respectively. This transformation demonstrates GVL’s capacity to support SNAr reactivity while offering a greener alternative to conventional solvents such as DMF or NMP.
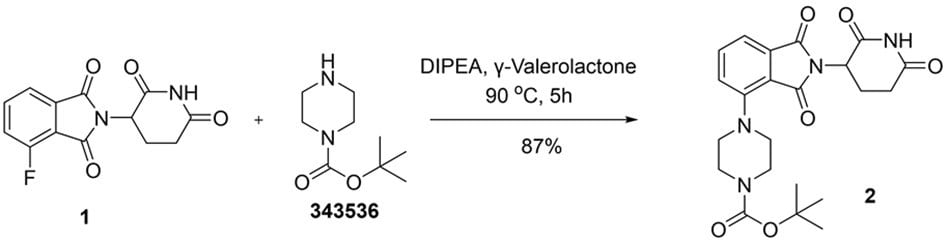
Figure 2.Synthesis of tert-butyl 4-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)piperazine-1-carboxylate (2) through nucleophilic aromatic substitution (SNAr) reaction of 2-(2,6-dioxopiperidin-3-yl)-4-fluoroisoindoline-1,3-dione (1) and 1-Boc-piperazine in GVL.
N-Alkylation
Substitution reactions involving alkyl halides and other leaving groups are commonly performed in toxic dipolar aprotic solvents such as DMF, NMP and DMSO, often in the presence of phase-transfer catalysts. In this context, GVL has been demonstrated as a viable, greener alternative, supporting efficient nucleophilic substitution without the need for hazardous media. We report two representative examples conducted in GVL, both yielding over 80%.
In the first case, 7-benzyl 2-(tert-butyl) 2,7-diazaspiro[3.5]nonane-2,7-dicarboxylate (4) was synthesized via the substitution of tert-butyl 2,7-diazaspiro[3.5]nonane-2-carboxylate (3) with 1-benzyl-2-bromoacetate (245631) in GVL in 98% yield compared to 76% yield in DMF.
In the second example, 3-(5-nitro-1-oxo-2,3-dihydro-1H-isoindol-2-yl)piperidine-2,6-dione (7) was obtained in 83% yield via the reaction of 2-(bromomethyl)-4-nitrobenzoate (5) with 3-aminoglutarimide hydrochloride (6), also in GVL. A comparable yield of 82% was obtained when the reaction was carried out in DMF.
Notably, both diazaspiro scaffolds and thalidomide8 derivatives are privileged structures in medicinal chemistry, frequently explored for their therapeutic potential in central nervous system (CNS) disorders, oncology, and immunomodulation. The successful execution of such transformations in GVL underscores its promise as a more sustainable solvent for drug discovery and development workflows.
![Synthesis of tert-butyl 7-(2-(benzyloxy)-2-oxoethyl)-2,7-diazaspiro[3.5]nonane-2-carboxylate 4 via substitution reaction between tert-butyl 2,7-diazaspiro[3.5]nonane-2-carboxylate 3 and 1-benzyl-2-bromoacetate in GVL. Synthesis of tert-butyl 7-(2-(benzyloxy)-2-oxoethyl)-2,7-diazaspiro[3.5]nonane-2-carboxylate 4 via substitution reaction between tert-butyl 2,7-diazaspiro[3.5]nonane-2-carboxylate 3 and 1-benzyl-2-bromoacetate in GVL.](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/articles/chemistry-and-synthesis/reaction-design-and-optimization/figure-3-example-n-alkylation-reaction/figure-3-example-n-alkylation-reaction.jpg)
Figure 3.Synthesis of tert-butyl 7-(2-(benzyloxy)-2-oxoethyl)-2,7-diazaspiro[3.5]nonane-2-carboxylate (4) via substitution reaction between tert-butyl 2,7-diazaspiro[3.5]nonane-2-carboxylate (3) and 1-benzyl-2-bromoacetate in GVL.
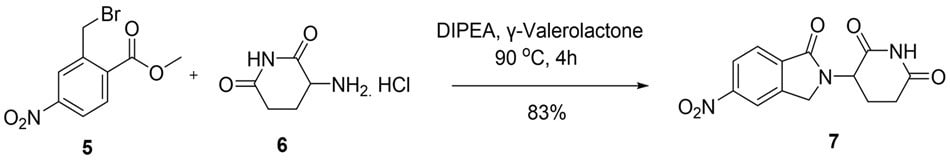
Figure 4.Synthesis of 3-(5-nitro-1-oxoisoindolin-2-yl)piperidine-2,6-dione (7) through substitution reaction of 2-(bromomethyl)-4-nitrobenzoate (5) and 3-aminoglutarimide hydrochloride (6) in GVL.
Amidation Reaction
Amidation reactions are fundamental to pharmaceutical and peptide synthesis, enabling the formation of amide bonds that are ubiquitous in drug molecules and bioactive compounds. Traditionally, these reactions are conducted in polar aprotic solvents such as DMF, NMP and DCM, which provide excellent solvation for carboxylic acids, amines, and coupling reagents. However, these solvents are associated with significant health and environmental concerns: they are reprotoxic, poorly biodegradable and increasingly subject to regulatory restrictions.
In response to these challenges, recent studies9 have explored the use of bio-based alternatives, such as GVL, which has shown promising compatibility with commonly used coupling agents. Although direct investigations of GVL with specific agents like HATU, EDC and DIC remain limited, emerging evidence supports its potential in greener amidation protocols. For example, a study10 evaluating the stability of coupling reagents in various solvents found that COMU®, a widely used coupling agent, retained 84% of its integrity in GVL after 48 hours, in contrast to its complete degradation in DMF over the same period.
Moreover, GVL has demonstrated successful application in solid-phase peptide synthesis (SPPS), particularly in the anchoring of amino acids onto Wang resins, where it achieved high loading efficiencies with minimal racemization. These findings underscore GVL's suitability for peptide synthesis workflows and suggest its broader applicability in solution-phase amidation reactions as well. Importantly, GVL's ability to preserve the stability of certain coupling reagents over extended durations represents a crucial advantage in multi-step synthesis settings.
While further research is needed to comprehensively establish GVL’s compatibility with a range of coupling agents -including HATU, EDC and DIC - current findings position it as a strong candidate for replacing conventional, hazardous solvents in amidation chemistry.11 To support this, some in-house examples were carried out wherein 2-[2-(tert-butoxycarbonylamino)ethoxy]acetic acid (8) was employed as the carboxylic acid component. In the first case, coupling with the aliphatic amine (S,R,S)-AHPC hydrochloride (901490) afforded the desired amide (9) in an 84% yield (Figure 5), whereas the same reaction afforded only 28% yield when conducted in DMF, further illustrating GVL's superior performance in practical amidation scenarios.
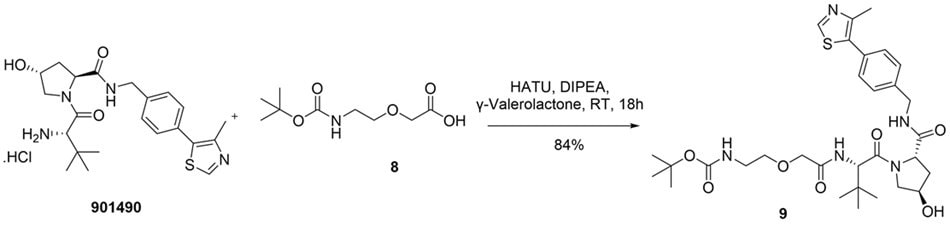
Figure 5.Synthesis of tert-butyl (2-(2-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-2-oxoethoxy)ethyl)carbamate (9) from (S,R,S) AHPC hydrochloride and 2-(2-((tert-butoxycarbonyl)amino)ethoxy)acetic acid (8) in GVL.
In a subsequent reaction, the same acid (8) was coupled with the less nucleophilic aromatic amine C5-lenalidomide (911690) , yielding the corresponding amide in 82% (Figure 6). These experiments demonstrate that amidation—a transformation traditionally reliant on toxic solvents—can be effectively executed in GVL, achieving a significantly improved yield compared to the literature result in DMF (57%)20 while also better aligning with Green Chemistry principles..

Figure 6.Synthesis of C5 Lenalidomide-PEG1-NH-Boc (10) from C5 Lenalidomide and 2-(2-((tert-butoxycarbonyl)amino)ethoxy)acetic acid (8) in GVL.
Further, we explored the synthesis of benzyl ((S)-2-hydroxypropanoyl)-L-phenylalaninate (11) via amide bond formation between (S)-2-hydroxypropanoic acid (L1750) and benzyl L-phenylalaninate (78060) in GVL (Figure 7). Although the yield was lower (36%) compared to other amidation reactions performed in our laboratory, this transformation is noteworthy due to the structural complexity of the substrates. Specifically, the reaction occurs between a secondary amine and a sterically hindered, secondary carboxylic acid, making it a challenging system. In comparison, the same reaction conducted in DMF yielded 60%. Importantly, this example also highlights amide bond formation occurring selectively over esterification, emphasizing GVL’s potential to support chemoselective transformations.
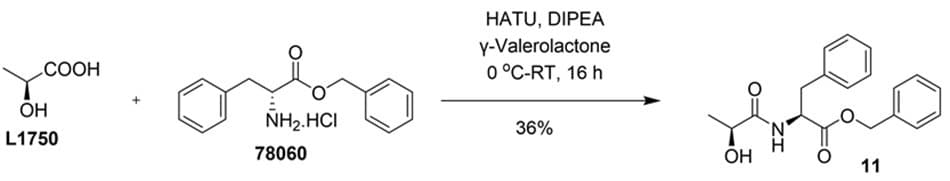
Figure 7.Synthesis of benzyl ((S)-2-hydroxypropanoyl)-L-phenylalaninate (11) from (S)-2-hydroxypropanoic acid and benzyl L-phenylalaninate in GVL.
In another study, we evaluated the coupling of an activated carboxylic acid, namely (+)-Biotin N-hydroxysuccinimide ester (H1759), with N-Boc-1,4-butanediamine (15404) in GVL (Figure 8). The reaction proceeded efficiently to afford the corresponding amide in 80% yield. This transformation serves as a representative example of a chemical biology application, where such biotinylated products are widely used in antibody–drug conjugates (ADCs) and drug discovery research. It is of note that although the 80% yield we report here is slightly lower than the literature report in DMF (85%)19, the reaction was run at room temperature as opposed to 100 °C.
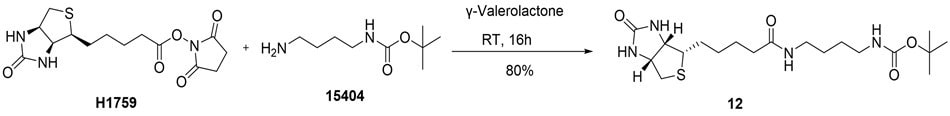
Figure 8.Synthesis of tert-butyl (4-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)butyl)carbamate (12) from (+)-Biotin N-hydroxysuccinimide ester and N-Boc-1,4-butanediamine in GVL
Coupling Reactions
Cross-coupling reactions are essential synthetic methodologies for constructing a wide range of carbon–carbon and carbon–heteroatom bonds, playing a pivotal role in the synthesis of natural products, pharmaceuticals, agrochemicals and advanced materials.12 Traditionally, these reactions are carried out in solvents such as NMP, DMF, 1,4-dioxane, toluene, MeCN, THF and dimethoxyethane, which offer strong solvation but pose significant health, environmental, and regulatory concerns. These challenges have intensified interest in greener solvent systems as part of more sustainable synthetic development13. Efforts to identify more sustainable alternatives include solvent screening studies and greener solvent selection guides. For example, Peng Lei14 et al. utilized such a framework to carry out Suzuki–Miyaura couplings of amides in greener solvents like tert-butyl methyl ether (MTBE), cyclopentyl methyl ether (CPME), diethyl carbonate (DEC), p-cymene, dimethyl carbonate (DMC) and anisole, replacing traditional dipolar aprotic and chlorinated solvents. In line with these efforts, we evaluated GVL as a more sustainable solvent for palladium-catalyzed cross-coupling reactions. Building on previous reports, including our earlier reference to the work by Strappaveccia4c et al., which demonstrated the utility of GVL in Heck–Mizoroki couplings, we applied this greener solvent in our own synthetic experiments. To our delight, tert‐butyl N‐{[4‐(4‐methyl‐1,3‐thiazol‐5‐yl)phenyl]methyl}carbamate (14) was synthesized via Heck coupling between tert-butyl (4-bromobenzyl)carbamate (13) and 4-methylthiazole (193925) in GVL, affording the desired product in an 82% yield after only 6 hours.
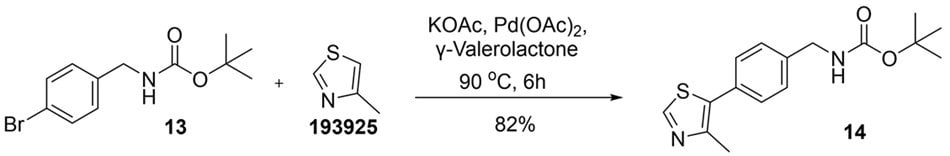
Figure 9.Synthesis of tert‐butyl (4-(4-methylthiazol-5-yl)benzyl)carbamate (14) from tert-butyl (4-bromobenzyl)carbamate (13) and 4-methylthiazole in GVL.
Reaction Summary
Several representative reactions were carried out in the lab to evaluate the performance of GVL as a greener solvent compared to traditionally used hazardous solvents. The results, summarized in Table 3, demonstrate GVL’s broad applicability across multiple reaction classes.
Notably, in a SNAr reaction, where conventional protocols rely on NMP or DMF - GVL enabled an equivalent or superior yield, confirming its capability to replace dipolar aprotic solvents in demanding transformations. Similarly, in amide bond formation, GVL performed exceptionally well under the same time and temperature conditions, delivering improved yields over standard solvents. However, limitations were observed in SNAr reactions involving azide substitution. When attempting to displace chloride with azide under basic conditions, no desired product was formed, likely due to nucleophilic attack of the azide anion on the lactone ring of GVL, leading to solvent decomposition. Despite this limitation, the overall performance of GVL across most reaction types was highly encouraging and supports its adoption as a safer, more sustainable solvent in synthetic workflows.
While GVL offers substantial benefits, challenges such as cost-effective large-scale production and global supply integration still hinder its widespread use. However, innovations in reactive distillation and hybrid catalytic pathways are making GVL increasingly viable at commercial scale. As regulatory pressure mounts on toxic solvents, GVL is poised to play a critical role in advancing greener industrial practices.
Conclusion
This application note establishes GVL as a versatile and high-performing bio-based solvent capable of replacing traditional dipolar aprotic solvents such as DMF, NMP, and DMAc across a wide range of synthetic transformations. Through both literature review and in-house experimentation, GVL demonstrated excellent performance in key reaction classes including SNAr, N-alkylation, amidation and Pd-catalyzed coupling.
GVL consistently delivered comparable or superior yields under similar conditions, as summarized in Table 3, reinforcing its synthetic efficacy. Notably, it enabled several complex and high-value reactions, including the formation of privileged medicinal scaffolds and peptide-like amides - highlighting its relevance in drug discovery, chemical biology, and materials science.
Beyond reactivity, GVL offers significant operational advantages: low toxicity, high thermal stability, ease of handling, low volatility, and minimal residual contamination. These features make it suitable for both bench-scale and process-scale applications. While limitations were observed in lactone ring degradation under harsh conditions - such cases are exceptions rather than the norm.
As regulatory pressure intensifies on toxic solvents and innovations in biomass valorization improve GVL’s commercial viability, this solvent is well-positioned to become a mainstream component of greener synthetic chemistry. Its alignment with Green Chemistry principles, combined with robust performance across diverse reaction types, supports its broader adoption in both academic and industrial settings.
References
Appendix
General Experimental Information
All commercially available reagents and solvents were used as received without further purification unless otherwise stated. Reactions were carried out under an inert atmosphere of nitrogen or argon in flame-dried or oven-dried glassware. Solvents used for reactions and chromatography were dried using standard procedures where required. Reaction progress was monitored by thin-layer chromatography on pre-coated silica gel plates (TLC aluminum sheets, Silica gel 60 F₂₅₄), and compounds were visualized using UV light or appropriate staining agents. Flash column chromatography was performed using silica gel (100–200 mesh). Organic solvents were removed under reduced pressure using a rotary evaporator, and products were dried under high vacuum. Purity and identity of compounds were confirmed by spectroscopic and analytical techniques. Nuclear magnetic resonance (NMR) spectra were recorded on a 400 MHz Bruker spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) relative to the residual solvent peak. Coupling constants (J) are reported in hertz (Hz). Melting points were determined using a Buchi apparatus and are uncorrected. Specific synthetic procedures and characterization data for each compound are detailed below.
Experimental Procedures
Synthesis of tert-butyl 4-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)piperazine-1-carboxylate (2) (Figure 2)
In a 100 mL round-bottom flask equipped with a nitrogen inlet and a condenser, 2-(2,6-Dioxo-3-piperidinyl)-4-fluoro-1H-isoindole-1,3(2H)-dione (1) (1.00 g, 3.62 mmol, 1.0 equiv) and N-Boc-piperazine 343536 (0.809 g, 4.34 mmol, 1.20 equiv.) were added to γ-valerolactone (933856) (15 mL). The resulting suspension was stirred for 10 minutes, after which N,N-diisopropylethylamine D125806 (1.40 g, 3.0 equiv., 10.9 mmol) was added dropwise. The reaction mixture was then heated to 90 °C and stirred for 5 hours. During the reaction, the suspension gradually turned from grayish to yellow. After completion, the mixture was cooled to room temperature, resulting in precipitation of the product. The solid was collected by filtration using a Büchner funnel, washed with water (2 X 50 mL), and dried to yield the target compound, 1,1-dimethylethyl-4-[2-(2,6-dioxo-3-piperidinyl)-2,3-dihydro-1,3-dioxo-1H-isoindol-4-yl]-1-piperazinecarboxylate (2) (1.40 g, 87%), as a yellow solid. 1H NMR (CDCl3, 400 MHz): δ 8.00 (s, 1H), 7.53 (t, J = 8.0 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.11 (d, J = 8.0 Hz, 1H), 4.87 (dd, J = 8.0 Hz, 1H), 3.58 (t, J = 8.0 Hz, 4H), 3.21 (t, J = 8.0 Hz, 4H), 2.81-2.66 (m, 3H), 2.04 (m, 1H), 1.43 (s, 9H) ppm.
Synthesis of tert-butyl 7-(2-(benzyloxy)-2-oxoethyl)-2,7-diazaspiro[3.5]nonane-2-carboxylate (4) (Figure 3)
A 100 mL three-neck round-bottom flask was equipped with a nitrogen inlet and charged with tert-butyl 2,7-diazaspiro[3.5]nonane-2-carboxylate (3) (1.0 g, 4.42 mmol, 1.0 equiv.) in γ-valerolactone (933856) (20 mL). To this solution, potassium carbonate (791776) (1.83 g, 13.26 mmol, 3.0 equiv.) was added and the mixture was stirred for 10 minutes. Benzyl 2-bromoacetate (245631) (1.21 g, 1.2 equiv, 5.30 mmol) was then added, and the reaction mixture was stirred at room temperature for 4 h. After completion, the reaction was quenched by pouring into ice water and extracted with ethyl acetate (3 X 100 mL). The combined organic layers were washed with saturated NaHCO₃ solution (30 mL) and brine (50 mL), then dried over anhydrous sodium sulfate. The solution was filtered and concentrated under reduced pressure to afford the crude product. Purification was carried out by column chromatography on silica gel (100–200 mesh), eluting with 15–20% ethyl acetate in hexane. The desired product, 7-benzyl 2-(tert-butyl) 2,7-diazaspiro[3.5]nonane-2,7-dicarboxylate (4) (1.62 g, 98%), was obtained as a white solid. TLC: Rf 0.53 in 30% ethyl acetate in n-hexane. visualized by heating with ninhydrin stain. 1H NMR (CDCl3, 400 MHz): δ 7.32-7.25 (m, 5H), 5.09 (s, 2H), 3.53 (s, 4H), 3.20 (s, 2H), 2.45 (s, 4H), 1.73 (t, J = 6.0 Hz, 4H), 1.37 (s, 9H) ppm.
Synthesis of 3-(5-nitro-1-oxoisoindolin-2-yl)piperidine-2,6-dione (7) (Figure 4)
To a stirred solution of 2-(bromomethyl)-4-nitrobenzoate (5) (2.74 g, 1.0 mmol, 1.0 equiv) and 3-aminoglutarimide hydrochloride (6) (1.98 g, 1.20 mmol, 1.2 equiv) in γ-valerolactone (933856) (15 mL), N,N-diisopropylethylamine (D125806) (0.52 g, 0.7 mL, 4.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred at 90 °C for 4 h. After completion, the mixture was cooled to room temperature and diluted with water (30 mL). The resulting precipitate was filtered, washed with water, and dried to afford 3-(5-nitro-1-oxoisoindolin-2-yl)piperidine-2,6-dione (7) (2.40 g, 83%) as a bluish-gray solid. TLC: Rf 0.60 in 10% methanol in dichloromethane, visualized under UV light (fluorescent and UV-active). 1H NMR (DMSO-d6, 400 MHz): δ 11.06 (brs, 1H), 8.54 (s, 1H), 8.36 (dd, J = 2.1, 8.4 Hz, 1H), 7.98 (d, J = 8.3 Hz, 1H), 5.16 (dd, J = 5.1, 13.3 Hz, 1H), 4.44 – 4.66 (m, 2H). 2.85 – 2.98 (m, 1H), 2.57 – 2.69 (m, 1H), 2.36 – 2.47 (m, 1H), 2.01 – 2.10 (m, 1H) ppm.
Synthesis of tert-butyl (2-(2-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-2-oxoethoxy)ethyl)carbamate (9) (Figure 5)
In a 50 mL three-neck round-bottom flask equipped with a nitrogen inlet, 2-[2-[[(1,1-dimethylethoxy)carbonyl]amino]ethoxy]acetic acid (8) (0.26 g, 1.19 mmol, 1.2 equiv) and HATU (445460) (0.566 g, 1.49 mmol, 1.5 equiv) were dissolved in γ-valerolactone (933856) (10 mL). The solution was stirred at room temperature for 10 minutes, then cooled to 0 °C using an ice-water bath. To this, (S,R,S)-AHPC hydrochloride (901490) (0.50 g, 0.99 mmol, 1.0 equiv) was added, followed by N,N-diisopropylethylamine (D125806) (0.52 mL, 2.97 mmol, 3.0 equiv). The reaction mixture was stirred at room temperature for 18 h. Upon completion, it was poured into ice-cold water and extracted with ethyl acetate (100 mL). The organic layer was washed with saturated NaHCO₃ solution (50 mL) and brine (50 mL), then dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to yield the crude product. The crude residue was purified by column chromatography on silica gel (100–200 mesh) using 2–3% methanol in dichloromethane as eluent to afford the product as a colorless sticky solid tert-butyl (2-(2-(((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidin-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)amino)-2-oxoethoxy)ethyl)carbamate (9) (0.53 g, 84%). TLC: Rf 0.7 in 5% methanol in dichloromethane. 1H NMR (DMSO-d6, 400 MHz): δ 9.05 (s, 1H), 8.65 (t, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.50-7.46 (d, J = 8.0 Hz, 4H), 6.95 (t, J = 8.0 Hz, 1H), 5.22 (brs, 1H), 4.61 (d, J = 8.0 Hz, 1H), 4.45-4.35 (m, 3H), 4.32-4.28 (m, 1H), 4.01 (s, 2H), 3.72-3.65 (m, 2H), 3.51 (t, J = 8.0 Hz, 2H), 3.19-3.17 (t, J = 8.0 Hz, 2H), 2.51 (s, 3H), 2.11 (m, 1H), 1.97 (m, 1H), 1.43 (s, 9H), 1.01 (s, 9H) ppm.
Synthesis of C5 Lenalidomide-PEG1-NH-Boc [tert-butyl (2-(2-((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindolin-5-yl)amino)-2-oxoethoxy)ethyl)carbamate] (10) (Figure 6)
In a 50 mL, three neck RB flask equipped with nitrogen inlet, 2-[2-[[(1,1-Dimethylethoxy)carbonyl]amino]ethoxy]acetic acid (8) (0.26 g, 1.2 mol, 1.20 equiv) and HATU (445460) (0.57 g, 1.49 mol, 1.5 equiv) were added in 10 mL γ-Valerolactone (933856). The solution was stirred for 10 minutes at room temperature to dissolve and the cooled to 0 oC using ice cold water and C5 Lenalidomide (911690) (0.5 g,, 0.99 moles, 1.0 equiv) was added, followed by addition of N,N-diisopropylethyl amine (D125806) (0.5 mL, 3.0 equiv., 2.90 moles). The reaction mixture was stirred at room temperature for 18h, after completion (checked by TLC; Rf 0.58 in 7% methanol in dichloromethane) poured into ice cold water. Reaction mixture was precipitated out and the resulting precipitate was collected by filtration through buchner funnel. The residual solid was washed with water (50 mL X 2) and dried to afford the product as a gray solid C5 Lenalidomide-PEG1-NH-Boc (10) (0.73 g, 82%). 1H NMR (DMSO-d6, 400 MHz): δ 10.99 (s, 1H), 9.95 (s, 1H), 8.03 (s, 1H), 7.73 (m 2H), 6.99 (s, 1H), 5.10 (dd, J = 4.0 Hz, 1H), 4.43-4.29 (dd, J= 4.0 Hz, 2H), 4.11 (s, 2H), 3.52 (t, J = 4.0 Hz, 2H), 3.18 (t, J = 6.0 Hz, 2H), 2.95 (m, 1H), 2.51 (m, 1H), 2.37 (m, 1H), 2.01 (m, 1H), 1.43 (s, 9H) ppm.
Synthesis of benzyl ((S)-2-hydroxypropanoyl)-L-phenylalaninate (11) (Figure 7)
In a 100 mL three-neck round-bottom flask equipped with a nitrogen inlet, (S)-2-hydroxypropanoic acid (L1750) (1.0 g, 11.10 mmol, 1.0 equiv) was dissolved in γ-valerolactone (933856) (20 mL) and cooled to 0 °C. To this solution, HATU (445460) (5.06 g, 13.32 mmol, 1.2 equiv) was added, and the mixture was stirred for 10 minutes at the same temperature. Subsequently, benzyl L-phenylalaninate (78060) (3.24 g, 11.10 mmol, 1.0 equiv) was added, followed by N,N-diisopropylethyl amine (D125806) (9.82 mL, 55.50 mmol, 5.0 equiv) dropwise via an addition funnel over 10–15 minutes at 0 °C. The light brown reaction mixture was then allowed to stir at room temperature for 16 hours. After completion, the reaction was poured into ice-cold water (20 mL) and extracted with ethyl acetate (2 × 50 mL). The combined organic layers were washed with saturated brine (10 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to yield a crude amorphous solid. The crude residue was purified by column chromatography on silica gel (100–200 mesh) using 30–40% ethyl acetate in hexane as eluent. Pure fractions were combined and concentrated to afford the product as a white solid benzyl ((S)-2-hydroxypropanoyl)-L-phenylalaninate (11) (1.32 g, 36%). TLC: Rf 0.4 in 50% EtOAc in hexane. 1H NMR (CDCl3, 400 MHz): δ 7.35-7.30 (m, 3H), 7.29-7.24 (m, 2H), 7.16-7.08 (m, 3H), 6.96-6.93 (m, 2H), 6.87-6.85 (m, 1H), 5.09 (q, 2H, J = 12.0 Hz), 4.87-4.82 (m, 1H), 4.14-4.09 (m, 1H), 3.13-3.00 (m, 2H), 1.27 (d, 3H, J = 4 Hz).
Synthesis of tert-butyl (4-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)butyl)carbamate (12) (Figure 8)
A 3-neck, 50 mL round-bottom flask was equipped with a nitrogen bubbler, and a solution of (+)-biotin N-hydroxysuccinimide ester (H1759) (1.0 g, 2.93 mmol, 1.0 equiv) in γ-valerolactone (933856 (30 mL) was added. The mixture was stirred until a clear solution was obtained. Then, N-Boc-1,4-butanediamine (15404) (0.61 g, 3.22 mmol, 1.1 equiv) was added, and the reaction mixture was stirred at room temperature for 18 h. After completion, diethyl ether (100 mL) was added, and the mixture was stirred for an additional 30 minutes. The resulting precipitate was filtered through a Büchner funnel and washed with ethyl acetate (25 mL). The residue was dried for 4 h to afford tert-butyl (4-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)butyl)carbamate (12) (0.97 g, 80%) as a white solid. TLC: Rf 0.38 in 10% methanol in dichloromethane; Visualization by PMA stain. 1H NMR (DMSO-d6, 400 MHz): δ 7.76-7.74 (t, J = 8.0 Hz, 1H), 6.80-6.78 (t, J = 8.0 Hz, 1H), 6.37 (s, 1H), 6.32 (s, 1H), 4.32 (t, J = 8.0 Hz, 1H), 4.12 (t, J = 8.0 Hz, 1H), 3.11-2.82 (m, 6H), 2.56 (d, J = 12 Hz, 1H), 2.06 (t, J = 8.0 Hz, 2H), 1.62-1.27 (m, 19H) ppm.
Synthesis of tert-butyl (4-(4-methylthiazol-5-yl)benzyl)carbamate (14) (Figure 9)
To a stirred solution of tert-butyl(4-bromobenzyl)carbamate (13) (2.0 g, 6.99 mmol, 1.0 equiv) in γ-valerolactone (933856) (20 mL) at ambient temperature, 4-methylthiazole (193925), (1.39 g, 13.98 mmol, 2.0 equiv) and potassium acetate (P1147) (1.37 g, 13.98 mmol, 2.0 equiv) were added. Nitrogen was bubbled through the reaction mixture for 10 minutes, after which palladium(II) acetate 205869 (0.157 g, 0.699 mmol, 0.1 equiv) was added. Then the reaction mixture was heated to 90 °C and stirred for 6 h. Upon completion, the reaction was cooled to room temperature, poured into water (100 mL), and extracted with ethyl acetate (250 mL). The organic layer was washed with water (100 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The crude residue was purified by column chromatography on silica gel (100–200 mesh) using hexane-ethyl acetate as the eluent to yield tert-butyl (4-(4-methylthiazol-5-yl)benzyl)carbamate (14) as a white powder (1.74 g, 82%). TLC: Rf 0.80 in 30% ethyl acetate in hexane. Visualisation through 254 nm UV light in chamber. 1H NMR (CDCl3, 400 MHz): δ 8.62 (s, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 4.86 (brs, 1H), 4.28 (d, J = 8.0 Hz, 2H), 2.46 (s, 3H), 1.40 (s, 9H) ppm.
如要继续阅读,请登录或创建帐户。
暂无帐户?