Chitosan in Pharmaceutical Applications
Chitosan is a non-toxic, biodegradable, and biocompatible polysaccharide derived from chitin, typically sourced from the exoskeletons of crustaceans. This natural polymer has found extensive use in various medical fields, particularly in tissue engineering, wound healing, drug delivery, and medical devices.
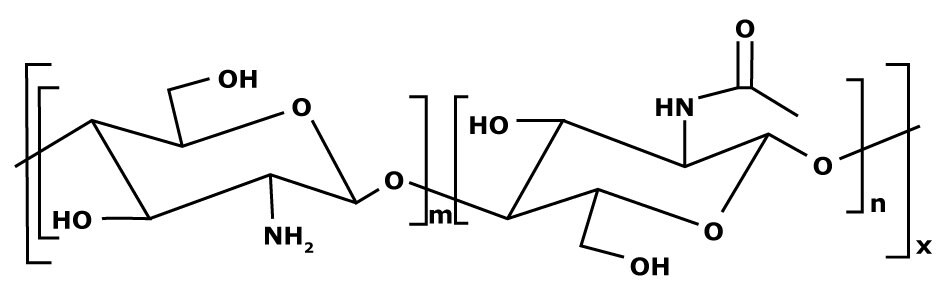
Figure 1. Chemical structure of chitosan.
High Purity Chitosan
High purity chitosan is a biomedical research grade, high molecular weight chitosan produced by Recirculating Aquaculture System (RAS) method by farming European spiny lobsters (Panulirus elephas) in a fully controlled, land-based environment. This method prevents any quality and batch-to-batch reproducibility inconsistencies, and sustainability issues. Chitosan is the only third-generation product with high molecular weight, reproducibility, and no detectable protein content, making it ideal for advanced biomedical therapies. It is used for bone, cartilage, and cardiac tissue regeneration, corneal and periodontal tissue regeneration, wound healing and gene delivery and bioimaging, vaccination, and cosmeceutical applications.
RAS Process
RAS system mimics natural habitats, achieving growth rates up to five times faster than wild lobsters, without the use of antibiotics, hormones, or chemicals. By maintaining optimal environmental conditions and implementing stress-reduction techniques, this process eliminates the risks associated with open-water farming. Chitosan is exclusively extracted from naturally molted shells, leaving lobsters completely unharmed. High purity chitosan uses an AI-based traceability system that tracks every molting cycle and efficiently collects the shed carapaces, ensuring ethical treatment of the lobsters and full traceability of every batch. Controlled deacetylation and cutting-edge purification processes is used to optimize chitosan properties for specific biomedical applications. These processes provide high purity and safety, with standardized procedures that ensure consistent quality across batches. Chitosan provides FDA and EMA-compliant chitosan for enhanced therapeutic efficacy in various applications. Biomedical chitosan stands out in the market due to its high purity, consistent quality, and traceability. With no detectable residual proteins, our chitosan eliminates potential inflammatory responses, offering maximum safety for medical applications.


Chitosan in Tissue Engineering
Chitosan's forms porous scaffolds that make it ideal for promoting cell growth and tissue regeneration. Its three-dimensional structure can be seeded with bioactive agents, including growth factors, to enhance tissue repair. The hemostatic and antimicrobial properties of chitosan helps in wound dressing applications, where it can limit infection risks and improve skin regeneration.
Chitosan in Drug Delivery
Chitosan is used as a carrier for controlled release of pharmaceuticals. It forms stable electrostatic complexes with polyanionic macromolecules, that can be used in gene therapy, for the encapsulation of DNA or RNA. It also improves solubility and buffering capacity for gene delivery to create amphiphilic derivatives for hydrophobic drug encapsulation.
Chitosan's primary amine groups allow for the formation of polyplexes for non-viral gene delivery, addressing challenges like nucleic acid degradation. Modifications such as quaternization improve solubility and stability, while grafting additional functional groups enhances transfection efficiency. These modifications enable effective delivery of therapeutic nucleic acids, including siRNA and DNA. To enhance stability and solubility, chitosan's primary amine can be quaternized, resulting in N,N,N-trimethyl chitosan chloride (TMC), which shows improved ionic complex stability. Further modifications, such as grafting thiols, enhance mucoadhesive properties, while converting amines to carboxylic acids increases solubility in neutral media, although stability with DNA may decrease. Grafting additional polymers or functional groups can further improve solubility and buffering capacity, with secondary and tertiary amino groups enhancing transfection efficiency through a simple one-step synthesis process.
Chitosan in Medical Devices
Due to its antimicrobial properties, chitosan is used for creating protective coatings and reduces the risk of device-associated infections. Its pH-sensitive nature allows the development of smart medical devices that can respond to environmental changes.
Preparation of Chitosan Microparticles by Spray Drying
Chitosan microparticles (diameter range of 1-10 µm) with controlled size and morphology are highly relevant in drug delivery and biopharmaceuticals. The size and the morphology of microparticles depends encapsulating bovine serum albumin (BSA), a model protein, and sodium tripolyphosphate (TPP), a cross-linker, and other processing parameters on the formation of particle size and morphology.
Chitosan with a molecular weight of 67,000 g/mole and varying formulations that included bovine serum albumin (BSA) and sodium tripolyphosphate (TPP) as a cross-linker, microparticles were created using a mini spray dryer. Scanning electron microscopy (SEM) revealed that the size and morphology of the microparticles remained largely unchanged with the addition of BSA or TPP; however, the average diameter increased from 3.2-3.5 µm to 4.3 µm when both were present. Lower chitosan concentrations resulted in more polydisperse particles with larger agglomerations. Additionally, spray drying parameters significantly impacted particle size, demonstrating the feasibility of tailoring microparticle characteristics for specific drug delivery applications.
Microparticles were prepared using a mini spray dryer (B-290) from BUCHI. The atomization parameters are reported in Table 2.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?