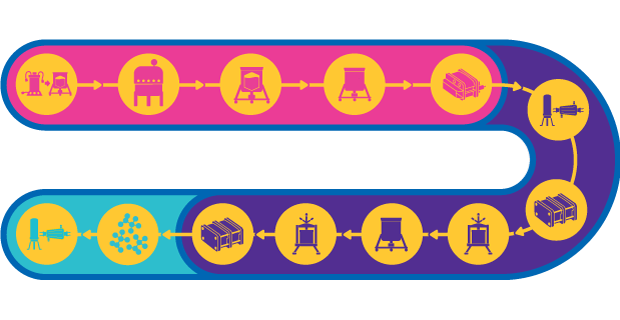
Virus-like Particles (VLPs) Vaccine Manufacturing
Virus-like particles (VLP) mimic the overall structure of virus particles but do not contain the infectious genetic material. When used as a vaccine, VLPs cause a robust immunogenic response due to their high-density display of epitopes and the capacity to present multiple proteins to the immune system.
Manufacturing of VLPs involves cell-based expression of the virus-shell protein. VLPs can be expressed in several heterologous expression systems including mammalian cell culture, baculovirus/ insect cell culture system, microbial fermentation and plants. VLPs are either assembled in vivo followed by purification from cell lysate, or the partially assembled protein is recovered from cell lysate and assembled into VLPs in vitro. Learn more about virus-like particle vaccine manufacturing.
Featured Categories
Our off-the-shelf and customizable bioprocessing cell culture media (CCM) products enhance productivity in upstream mAb, vaccine, gene/cell therapy processes.
The Mobius® Bioreactor family offers single-use bioreactors for cell culturing from early development to commercial production, supporting various scales.
Liquid formulations are an essential part of any pharmaceutical portfolio. As your ideal partner for liquid applications we provide excipients for both small molecule and large molecule liquid formulations...
Explore Mobius® single-use mixing solutions for pharmaceutical ingredient blending and process solution preparation.
Optimize upstream productivity and clarification with reliable scale-up
The upstream production platform selected for manufacturing of VLPs must be optimized to meet productivity requirements. This optimization includes the clarification step which follows cell lysis for removal of cells and cell debris and ensures a robust harvest of particles. The upstream process is only successful, however, if it can be reliably scaled in order to meet anticipated market demand.
Achieve yield and efficiency goals with robust impurity removal
Nucleic acids from lysed cells are a common contaminant in VLP processes. The European Medicines Agency (EMA) and World Health Organization (WHO) allow 10 ng DNA per dose for parenteral vaccines and 100 µg DNA per dose for oral vaccines. Additionally, in order to minimize the risk of host cell nucleic acid oncogenicity, DNA size must be reduced to 100–200 base pairs in length.
Maximize downstream recovery
VLPs are commonly purified by ultracentrifugation. While this process is well established for small-scale production, it can be time consuming and poorly scalable. Purification methods such as ion-exchange chromatography can be used as an alternative. In certain processes, membrane adsorption and monolith technology can provide better dynamic binding capacity (DBC) than particle-based resins. Multimodal resins that employ both size exclusion and binding-based separation are another option.
Ensure patient safety with sterile filtration, formulation and final fill
To help ensure patient safety, the final VLP product must be sterile filtered using a 0.22 µm filter. Formulation of VLP-based vaccine can be achieved using single-use components; single-use bags containing formulation reagents can be connected to any mixer through sterile quick-connects. After compounding and formulation, the product can be aseptically transferred to single-use filling systems for final filling and vialing.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Adjuvant selection for vaccine formulation considers safety, effectiveness, and other characteristics for optimal application.
- This technical article breaks down the steps of upstream and downstream bioprocessing and formulation of virus-like particle vaccines.
- Cost Modeling Vaccine Manufacturing: Estimate Production Costs for mRNA and other Vaccine ModalitiesA custom-designed cost model is used to explore the economics of vaccine manufacturing across several different modalities including mRNA. The model enables greater process understanding, simulates bottlenecks, and helps to optimize production efficiency.
- See All (0)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
Upstream Cell Culture
Maximize upstream productivity of VLPs and ensure robust scalability with:
- Cell Culture with CellPrime® rTrypsin
- EX-CELL® CD Insect Cell Medium
- Cell Culture with Mobius® Single-use Bioreactors
- Virus Filtration with Viresolve Barrier Micro Process Development Kit
- Sterile Sampling with NovaSeptum® GO Sterile Sampling System
- Sf-RVN® Insect Cell Line
- Upstream Process Chemicals
Nuclease Treatment & Clarification
Achieve the desired yield of VLPs and process efficiency while ensuring robust impurity removal with:
- Nuclease treatment with Benzonase® suitable for biopharmaceutical production EMPROVE® bio
- Benzonase® Detection with Benzonase® Elisa Kit II to detect left over Benzonase® endonuclease in the process
- Primary/Secondary Clarification with Millistak+® HC POD Depth Filters
- Primary Clarification with Clarisolve® Depth Filters
- Secondary Clarification with Polysep II cartridges
- Secondary Clarification with Milligard® PES filters
Downstream - Tangential Flow Filtration
Achieve yield, efficiency and virus recovery goals while ensuring robust impurity removal with:
- Ultrafiltration / Diafiltration with Pellicon® 2 Cassettes
- Ultrafiltration / Diafiltration with Pellicon® 3 Cassettes
- Ultrafiltration / Diafiltration with Pellicon® Capsule with Ultracel® Membrane
- Mobius® TFF 80 System, Mobius® FlexReady Solution for TFF, Cogent® Lab Systems and Cogent® Process-scale Tangential Flow Filtration System
Downstream - Chromatography
- Membrane Chromatography with Eshmuno® Q Resin
- Membrane Chromatography with Natrix® Q Recon Mini Chromatography Membranes
- Membrane Chromatography with Natrix® Q Pilot Chromatography Membrane
- Capture and/or Polishing Chromatography with Fractogel® EMD DEAE Chromatography Resins
- Capture and/or Polishing Chromatography with Fractogel® EMD DMAE Chromatography Resins
- Mobius® FlexReady Solution for Chromatography

Final Sterile Filtration & Filling
Ensure patient safety with reliable and robust sterile filtration, formulation and final fill.
- Final Sterile Filtration & Filling
- Durapore® 0.22 µm Filters
- Millipore Express® SHF (Sterile, High Flux) Filters
- Sterile Sampling with Novaseptum® Go Sterile Sampling Solutions
- Fill-Finish with Mobius® Single-Use Fill-Finish Solutions
- Sterile Filtration Strategies
- Final Fill Strategies
- Millipak® Final Fill Filters
- Mobius® 2D and 3D Assemblies and Storage Systems
Analytics Software & PAT Technology
To continue reading please sign in or create an account.
Don't Have An Account?