mRNA Preclinical Testing
Advancing mRNA Therapeutics and Vaccines Through Innovative Biological Modeling and Immune Profiling
Developing safe, effective mRNA-based therapeutics starts with reliable preclinical models. While 2D cell cultures serve as useful early tools, they often fail to capture the complexity of human biology. That’s why we integrate advanced 3D models, including patient-derived organoids (PDOs), to simulate more physiologically relevant responses and improve translational outcomes.
With exclusive access to patient-derived organoid biobanks and assay development services using HUB Organoid technology and our partnership with BioIVT for high-quality immune cell subsets, our portfolio enables researchers to explore mRNA function in systems that closely mirror human physiology. By combining patient-derived and immune-responsive models, we help bridge the gap between in vitro screening and clinical studies—reducing attrition rates and accelerating progress toward clinical success.
We also offer integrated immune profiling capabilities to assess both antibody and T-cell responses—providing critical insights into the effectiveness of mRNA-based interventions.
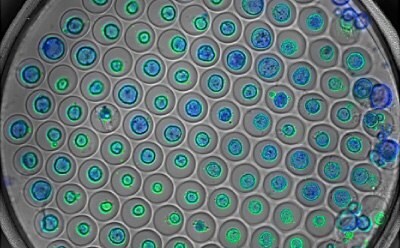
Organoids cultured and imaged in Millicell® Microwell Plates
Transforming mRNA Discovery with Predictive Biological Models
Patient-derived Organoids (PDOs)
Patient-derived organoids (PDOs) offer a powerful approach to in vitro preclinical assessment of mRNA-based therapeutics by recapitulating:
- Tissue-relevant mRNA expression: Patient-derived organoids retain the genetic characteristics of their original tissue including mRNA expression patterns, yielding crucial insights when profiling immune and drug responses.
- In vivo-like functional responses and pathway activations: Patient-derived organoids maintain the functional and molecular response of their source tissue to better elucidate the mechanism of action of drugs, determine key pathways involved in drug response or resistance, and identify potential biomarkers that predict drug response.
- Diversity: The ability of patient-derived organoids to recapitulate a targeted or diverse population supports better assessment of mRNA vaccine efficacy and safety across genetic backgrounds, disease subtypes, and even rare mutations, capturing variable responses earlier in development.
View our offering of HUB organoids or make a request.
Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs are vital for evaluating drug efficacy, safety, and immune response, and play a central role in the development of vaccines and immunotherapies. In partnership with BioIVT, we provide pre-isolated immune cells that can be selected based on specific donor criteria to meet your research needs.
- Vaccine Development: Assess how a vaccine candidate stimulates immune responses, such as antibody production.
- Efficacy and Safety Assessment: Evaluate interactions between therapeutic candidates and the immune system, including immune activation or suppression.
- Drug Target Validation: Identify immune cell subsets involved in diseases to help validate drug targets.
- Screening Assays: Use PBMCs to screen and optimize drug candidates.
- Biomarker Discovery: Leverage PBMCs as a source of biomarkers to predict disease progression.
Cell Cultureware for Reproducible Cultures
Support scalability with Millicell® Microwell Plates and Cell Culture Inserts:
- Millicell ® ULA Plates for reliable scaffold free self-assembly of spheroids
- Millicell® Cell Culture Inserts for more natural, multidimensional growth
Reliable Verification Tools for Confirmatory Studies
Ensure data accuracy with our suite of proven immunoassay and protein tools:
- ELISA Kits
- MILLIPLEX® Multiplex Immunoassays including nonhuman primate, rat, and mouse cytokine panels.
- Western Blot Analysis
- mPAGE® Lux Protein Gel Casting System
Together, these tools provide high-confidence verification of mRNA expression and downstream biological effects.
3D bioprinting
Generate precisely controlled 3D tissue constructs with tissue-like complexity
3D bioprinting offers a novel approach to in vitro preclinical assessment of mRNA-based therapeutics by providing:
- Enhanced tissue models: 3D bioprinting enables the creation of complex tissue structures that closely mimic native human tissues, providing a more accurate representation for assessing mRNA-based interventions.
- Customizable microenvironments: Precise control over the cellular composition and microenvironment within 3D bioprinted tissues facilitates the study of mRNA delivery, expression, and therapeutic responses in a physiological relevant context.
- High-throughput capabilities: utilizing 3D bioprinted tissue models allows for scalable and automated platforms, enabling efficient mRNA screening with increased throughput and improved reproducibility.
Innovation in mRNA Screening: Identifying the Right Candidates Early
Selecting optimal mRNA candidates is one of the most critical steps in developing successful therapeutics and vaccines. By prioritizing molecules that are both highly expressed and biologically active, researchers can streamline development and reduce downstream failures.
Power Up Primary Screening with scRNA-seq
Our advanced MULTI-seq technology enables simultaneous analysis of multiple samples, delivering a robust, multiplexed profile of gene expression. It’s designed to increase throughput and reproducibility while reducing cost and time.
Measuring Immune Response to mRNA Vaccines
Understanding the immune system’s response to mRNA vaccines is crucial for evaluating their effectiveness and ensuring long-lasting protection. Unlike traditional vaccines, mRNA vaccines instruct cells to produce specific proteins that trigger comprehensive immune responses involving both B cells and T cells. By leveraging advanced serological and cellular analysis tools, researchers can evaluate the strength and diversity of both antibody and T-cell responses to mRNA vaccines. This comprehensive insight helps determine how well a vaccine stimulates protective immunity, supporting the development of safer, more effective vaccine candidates, dosing regimens, and accelerating safe, effective vaccine development.
Humoral Immunity: Measuring Antibody Responses
B cells play a critical role in immune defense by producing antigen-specific antibodies that neutralize pathogens. Our serological capabilities, such as multiplex MILLIPLEX® immunoassays, enable precise quantification of antibody levels and isotype profiles. These insights help researchers evaluate mRNA vaccine efficacy, guiding decisions around booster timing, dosing strategies, and candidate selection.
- Multiplex Immunoassays: Simultaneous detection of multiple antibody isotypes for a comprehensive immune profile.
- Custom Assay Development and Innovation (CADI): Reproducible and scalable bespoke multiplex or high sensitivity immunoassays for your serological and immunological biomarker(s) of interest.
Evaluating T-Cell Immunity in mRNA Vaccine Development
While antibody production is essential for vaccine protection, T-cell–mediated immunity plays an equally critical role in ensuring long-term defense. mRNA vaccines are designed to activate both CD4⁺ helper T cells, which support antibody generation, and CD8⁺ cytotoxic T cells, which target and eliminate infected cells.
To assess the strength and specificity of the cellular immune response, we offer a suite of powerful tools and assays:
- ELISpot: Detect cytokine-secreting T cells (e.g., IFNγ, IL-2) in response to antigens.
- Multiplex Immunoassays: Profile multiple cytokines simultaneously (e.g., TNFα, IFNγ, IL-4, IL-5, IL-13) for a broad immune snapshot.
- Western Blot: Confirms expression of T-cell activation markers.
- qPCR: Measures gene expression changes linked to immune activation.
- Single-Cell RNA Sequencing (scRNA-seq): Reveals T-cell heterogeneity and activation at single-cell resolution.
- Duolink® PLA (Proximity Ligation Assay): Visualizes complex formation within cells and tissues, in both IHC and flow cytometry experiments.
Related Services
Proven expertise across multiple platforms to support your immunoassay requirements.
Related Categories
Primary and secondary antibodies and antibody conjugates for ELISA and immunoassay.
Medium and high-binding plates to develop your own ELISAs.
Filter plates for ligand binding assays, ELISpot assays, and more.
Featuring high-performance Millipore® membranes in a wide variety of formats.
Promoting natural, multidimensional growth for more relevant in vitro models.
Dependable reagents for breakthrough research, ensuring high-quality, reproducible results.
Cell health analysis reagents and kits to measure viability, proliferation, cytotoxicity, and more.
Related Articles
- IFN-γ ELISpot Assays on MultiScreen® IP
Detailed protocol and optimization for ELISpot assays.
- A Highly Sensitive IFN-γ ELISpot Assay to Quantify Cellular Immune Responses to Previous Viral Infection
Learn about ELISpot assays for evaluation of immune response.
- 3D Cell Culture Tools and Techniques
Select the best cell culture model for your studies.
- Organoid Culture Frequently Asked Questions
Learn tips and tricks for organoid culture and analysis.
- Growing Small Intestine Organoid-Derived Monolayers using Millicell® Inserts
Culturing organoids in cell culture inserts to monitor long-term drug side effects and toxicity.
Explore Our Products & Services
Find products and services to facilitate antigen discovery, mRNA synthesis, and lipid nanoparticle selection and formulation for biopharmaceutical and life science research applications.
Tools for Pathogen Analysis, Validation Tools and Reagents, and mRNA Design and Optimization
To continue reading please sign in or create an account.
Don't Have An Account?