How to Automate Duolink® Assays on the AAW™ Workstation
This protocol details how to automate Duolink® assays with the AAW™ automated assay workstation. These 96-well microplates enable enhanced walkaway time and high-throughput capabilities. View details and data below.

Intro to Automating Duolink® Assays
The AAW™ Automated Assay Workstation powered by Opentrons® is a modular robotic liquid handler designed for automating assay and sample prep processes. This application study describes the process of implementing Duolink® PLA kits on the AAW™ Workstation and the performance of the protocol to increase lab efficiency by minimizing hands-on time while still maintaining assay functionality and robustness.
Duolink® assays are proximity ligation assays (PLA) that are used to visualize protein-protein interactions in cells. Complementary DNA oligonucleotides are conjugated to antibodies specific to proteins of interest. Once proteins come in close contact, ligation and amplification steps of the oligonucleotides allow protein-protein complexes to be imaged. While this assay does not require extensive training, it requires many manual steps that impede its ability to scale up, and automation of this assay could help to improve lab efficiency.
Automation and Duolink® Materials Required
Labware
- Opentrons® 200 μL tip rack, filtered (Prod. No. 99100105)
- (Day 1: 1 box, Day 2: 3 or 4 boxes)
- Opentrons® 1,000 μL tip rack, filtered (Prod. No. 99100106)
- (Day 1: 1 box, Day 2: 2 boxes)
- NEST Deep Well 96-Well Plate (Opentrons® Prod. No. 99900103)
- NEST 12-Well Reservoir (Opentrons® Prod. No. 99900076)
- NEST 290 mL 1-Well Reservoir (Opentrons® Prod. No. 99900207)
- Assay Plate: Square Well (Ibidi® Prod. No. 89626 or 89627 is recommended) or Round Well (Corning® Prod. No. CLS3603 or similar)
Hardware
- AAW™ Automated Assay Workstation (Prod. No. 99900241)
- AAW™ 8-Channel Pipette, 1, 000 μL (Prod. No. 99900245)
- AAW™ 1-Channel Pipette, 1,000 μL (Prod. No. 99900246)
- AAW™ Flex Gripper (Prod. No. 99900244)
- Opentrons Flex™ Heater-Shaker (Get A Quote)
- Opentrons Flex™ Deck Expansion (4pk) (Prod No. 99900203)
- Optional: Opentrons Flex™ Temperature Module (Get A Quote)
Reagents
- Duolink® Blocking Solution (Prod. No. DUO82007)
- Primary Antibodies specific to target proteins
- Duolink® PLA Probes (5x) (one PLUS and one MINUS from different species, matching the host species of your primary antibodies)
- Antibody Diluent (Prod. No. DUO82008) – For dilution of PLA probes, and if needed, primary antibodies
- Ligation Stock (5x) – Diluted in high purity water, such as Milli-Q® lab water systems, to make 1x Ligation Buffer (Prod. No. DUO92008 or similar)
- Ligase (1 U/μL, 40x) – Diluted to 1x in Ligation Buffer to make the Ligation Solution (Prod. No. DUO92008 or similar)
- Amplification Stock (green, orange, red, and far red) (5x) (Prod. No. DUO92008 or similar) – Diluted in high purity water to make 1x Amplification Buffer
- Note: The data below used the red detection reagents
- Polymerase (10 U/μL, 80x) – Diluted to 1x in Amplification Buffer to make the Amplification Solution (Prod. No. DUO92008 or similar)
- Wash Buffers A and B (Prod. No. DUO82049)
- Should be made prior to beginning the assay by dissolving the contents of one pouch in high purity water to a final volume of 1,000 mL. Solutions may be stored at room temperature for short term storage (less than two weeks) or at 4 °C for long term storage.
- Note: Bring the solutions to room temperature before use.
- 0.01x Wash Buffer B
- Should be made prior to beginning the assay by diluting 1x Wash Buffer B 1:100 in high purity water.
- DAPI (10x) (Prod. No. DUO82064) - Diluted to 1x in high purity water
- Anti-Fade (10x) (Prod. No. DUO82064) - Diluted to 1x in high purity water
Duolink® Protocol Uploading
Download your chosen Duolink® PLA 96-well protocol from the Millipore® Protocol Library and upload to the Opentrons® app. Currently available protocols allow for choosing either a 1) Duolink® Component Testing protocol to test antibody concentrations or positive/negative controls or the 2) Duolink® Multiwell Plate assay protocol that allow customers to run up to 96 samples using a single chosen antibody pair combination and concentration. Each protocol comes in a square or round 96-well protocol version.
- Duolink® Component Testing Assay Day 1
- Duolink® Component Testing Assay Day 2
- Duolink® Multiwell Plate Day 1
- Duolink® Multiwell Plate Day 2
AAW™ Workstation Set-up
The AAW™ workstation was used to automate Duolink® PLA assays. Assay protocols were adapted into Python code and imported into the Opentrons® app onto the workstation. The instrument performed all liquid handling steps, incubation, and shaking steps. If the Temperature module option is chosen, all assay steps are walk away. If not, 3 brief manual intervention steps are required to add temperature sensitive reagents to the deck as needed.
Preparing the instrument for the Duolink® Assay
- Download the chosen Duolink® protocol file from the Millipore® Protocol Library and load in the Opentrons® App on your computer as described above. The protocol can then be sent to the AAW™ Workstation via the “Send to Opentrons FLEX” or “Start Set-up” functions.
- Ensure appropriate modules, fixtures, and pipettes are loaded into the appropriate locations and calibrated according to the AAW™ User Manual.
- Once protocol is sent to the robot, the assay can be initiated by selecting the protocol and “Start Set-up” button. A list of parameters will be shown whereupon specific assay parameters selections can be made. The operator can choose to alter, but default selections have been made:
Day 1
- Number of Samples: 1-96
- Note: only if Full Plate Protocol is chosen
- Dry Run: On or off (Default) for use as practice runs with shortened incubation and wash steps
- Incubation on Deck: On (Default), off
- Use Plate Lid: On (Default), off gives the option to have the AAW™ Flex gripper lid the plate (ON) or the user to manually lid the plate (off)
Day 2
- Number of Samples: 1-96
- Dry Run: On or Off (Default) for use as practice runs with shortened incubation and wash steps
- Incubation on Deck: On (Default), off
- Use Plate Lid: On (Default), off gives the option to have the AAW™ Flex gripper lid the plate (ON) or the user to manually lid the plate (off)
- Use Temperature Module: On (Default), off gives the option to have Day 2 completely walk-away. If not used, 3 brief manual intervention steps are required to add temperature sensitive reagents to the deck as needed.
- Prior to starting a run, a Labware Position Check must be performed at least once by following the step-by-step instructions on the touchscreen or in the Opentrons® app. Once labware offsets are confirmed, the AAW™ workstation stores these values for future runs and will not have to be repeated unless desired. Labware position check can be run with empty labware during a dry run or with reagent ready labware. Ensure the correct labware position offsets are used depending on if Temperature module is selected or not.
Duolink® Assay Set-Up Procedure
The Duolink® PLA was performed according to the product instructions for multiwell plates manually and by using the adapted protocol on the AAW™ workstation (see below). All steps of the assay were performed on the instrument deck. If the Temperature Module is selected, no manual intervention steps on Day 2 are required. If not selected, pauses are built into the protocol to add the temperature sensitive Ligase and Polymerase/Amplification Solutions. As mentioned above, each protocol comes in a square or round 96-well protocol version. Assays run in square wells require 80 µL per well of reagent volume and those in round well require 40 µL per well. 100RXN Duolink® kits are sufficient volume for round wells, however, additional reagents may need to be purchased to account for 80 µL well volume in square wells.
Preparation Prior to Run
- Culture cells in multiwell plates then treat (starve, stimulate etc.), wash, and fix the cells according to your standard immunostaining protocol. The conditions for your primary antibodies should be optimized with respect to sample fixation, antigen retrieval, blocking solution, antibody diluent, concentrations, and incubation temperature and time.
- For this EGFR/HER2 application, SK-OV3 cells were cultured in multiwell plates (square well and round well), stimulated with EGF, and fixed according to the instructions from the product info sheet for the Duolink® Control Kit and multiwell plate protocol.
- Prior to running the Duolink® protocol on the AAW™ workstation, cells must be permeabilized for 10 min (0.2% Triton X-100 (Prod. No. T8787) in 1´ PBS (Prod. No. P3813)) for primary antibodies to reach their intracellular targets.
- Wash cells twice in PBS for 2 min, gently shaking, and finally remove any excess PBS from the plate immediately prior to running the assay.
Day 1
Reagent Preparation
Please follow the Duolink® PLA fluorescence protocol for Day 1 reagent preparation.
- Prepare primary antibody dilutions for either the Duolink® Component Testing Assay or Duolink® Multiwell Plate Assay. The Duolink® Component Testing Assay allows for identifying positive/negative control antibody combinations or testing different antibody concentrations.
- For the EGFR/HER2 Duolink® Component Testing assay, primary antibodies (mouse anti-EGFR (Prod. No. E3138) and the rabbit anti- ErbB2/HER2 (Prod. No. HPA001383)) were diluted in Duolink® PLA antibody diluent (Prod. No. DUO82008) to final concentrations of 1:100 and 1:5,000, respectively. Antibody conditions tested were negative control -EGFR/-HER2, +HER2/-EGFR, -HER2/+EGFR, and positive control +HER2/+EGFR.
- For the 96-sample EGFR/HER2 Duolink® Multiwell Plate assay, primary antibodies (mouse anti-EGFR antibody and the rabbit anti- ErbB2/ HER2) were diluted in Duolink® PLA antibody diluent (Prod. No. DUO82008) to final concentrations of 1:100 and 1:5,000, respectively. A full plate of positive control +HER2/+EGFR primary antibodies was run.
- Antibodies are loaded into a NEST Deep Wall Reagent Plate according to Assay run type (Component Testing vs. Multiwell Plate).
- For Component Testing Assay: 4 sets of positive or negative control antibodies (or concentrations) are loaded into wells A1-D1.
- For Multiwell Plate Assay: Load a single antibody solution into wells A1-H1.
Note: Assay volumes for the Assay run types are displayed in the app or touch screen upon assay start up. If the sample number is other than 96, please refer to Opentrons® app or touch screen for exact volumes required.
- Duolink® Blocking Solution should be added to wells A2-H2 of the Reagent Plate. Volumes can be found on the app or touchscreen upon initiating the protocol.
Robot Deck Configuration and Automated Assay Protocol
- Once all reagents are placed in their appropriate wells on the NEST Deep 96-well Reagent plate, labware can be placed on to the deck in the appropriate locations as listed below and depicted in Figure 1 and Figure 2:
- 1x box of P200 tips in B2
- 1x box of P1000 tips in B3
- NEST Deep 96-well Reagent plate in C1
- Sample plate in C2
- Sample plate lid in C4
- NEST 290 mL Reservoir in D2

Figure 1. Day 1 Control Plate Deck.Deck map configurations for Day 1 of Duolink® Component Testing. Schematics of the AAW™ deck set-up configuration showing labware and hardware locations for Day 1 of Duolink® Component Testing.

Figure 2. Day 1 Full Plate Deck.Deck map configurations for Day 1 of Duolink® Multiwell Plate assays. Schematics of the AAW™ deck set-up configuration showing labware and hardware locations for Day 1 of Duolink® Multiwell Plate assays.
- Once all labware is in its proper location, the robot door can be closed. From there, the Start Set-Up button can be selected, parameters chosen, and Labware Position Check performed (if not done already) as described in the AAW™ Workstation Set-up section above. If the Start Set-up function has already been activated and parameters selected, the assay can begin by selecting the blue “Run arrow”.
- The AAW™ workstation will now execute the protocol.
- Once the program is complete, the assay plate is ready in C2 with added primary antibodies for off-deck overnight incubation at 4 °C.
Day 2
Reagent Preparation
If using the Temperature module, all solutions can be made at the start of the assay and maintained at 4 °C throughout the automated assay.
If not using the Temperature module, make the components immediately before use and add into the reagent wells during the built-in program pauses instructed to do so.
Please follow the Duolink® PLA fluorescence protocol for reagent preparation. Volumes can be found in the Opentrons® app or AAW™ touchscreen upon assay start-up.
- Place PLA Probes in wells A1-H1 of Reagent Deep well plate.
- Place the prepared ligase solution (1x ligase + 1x ligase buffer) in wells A2-H2 of Reagent Deep well plate.
- Place prepared amplification solution (1x polymerase + 1x amplification buffer) solution in wells A3-H3 of Reagent Deep well plate.
- Place 1x DAPI nuclear stain in wells A4-H4 of Reagent Deep well plate.
- Place 1x anti-fade in wells A5-H5 of Reagent Deep well plate.
- Wash Buffers
- Wash Buffer A
- Duolink® Component Testing Plate: Wells 1-6 of NEST 12-well Reservoir
- Duolink® Multiwell Plate: NEST 290 mL Reservoir
- Wash Buffer B
- Duolink® Component Testing Plate: Wells 7-8 of NEST 12-well Reservoir
- Duolink® Multiwell Plate: Wells 1-4 of NEST 12-well Reservoir
- Wash Buffer 0.1x B
- Duolink® Component Testing Plate: Well 9 of NEST 12-well Reservoir
- Duolink® Multiwell Plate: Wells 5-6 of NEST 12-well Reservoir
- PBS
- Duolink® Component Testing Plate: Well 10 of NEST 12-well Reservoir
- Duolink® Multiwell Plate: Wells 7-8 of NEST 12-well Reservoir
- Wash Buffer A
Robot Deck Configuration and Automated Assay Protocol
- Once all reagents are placed in their appropriate wells on the NEST Deep 96-well Reagent plate, labware can be placed onto the deck in the appropriate locations as listed below and depicted in Figure 3 and Figure 4:
- 4x boxes of P200 tips in A1, A2, B1, and B2 for Duolink® Component Testing assay or 3x boxes of P200 tips in A1, A2, B1 for Duolink® Multiwell Plate assay
- 2x boxes of P1000 tips in A3 and B3
- NEST Deep 96-well Reagent plate in C1 (with or without the Temperature module)
- Sample plate (square or round 96-well plate) in C2 and sample plate lid in C4
- Wash buffer NEST 12-well Reservoir in C3 for Duolink® Component Testing assay
- For Duolink® Multiwell Plate assay, wash buffer NEST 12-well Reservoir in B2 and NEST 290 mL Reservoir in C3
- Empty NEST 290 mL Reservoir in D2
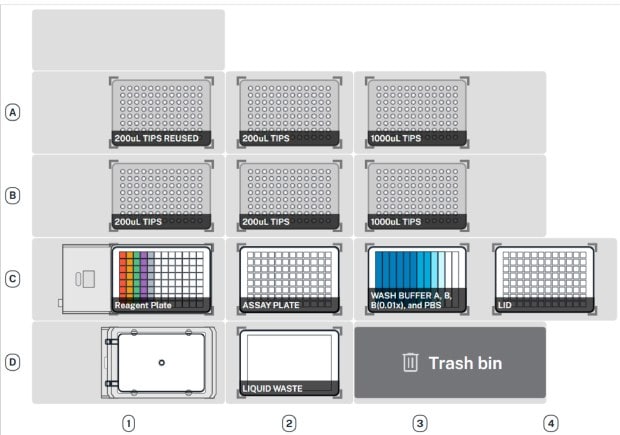
Figure 3. Day 2 Control Plate Deck.Deck map configurations for Day 2 of Duolink® Component Testing. Schematics of the AAW™ deck set-up configuration showing labware and hardware locations for Day 2 of Duolink® Component Testing.

Figure 4. Day 2 Full Plate Deck.Deck map configurations for Day 2 of Duolink® Multiwell Plate assays. Schematics of the AAW™ deck set-up configuration showing labware and hardware locations for Day 2 of Duolink® Multiwell Plate assays.
- If the Start Set-up function has already been activated and parameters selected, the assay can begin by selecting the blue “Run arrow”.
- The AAW™ workstation will now execute the protocol.
- If using the temperature module, no manual intervention is required.
- If not using the Temperature module, there are built-in protocol pauses with prompts for the user to add temperature sensitive reagents (ligase, polymerase, etc). The touchscreen will display the time to the next pause step so you can coordinate and walk away.
- Once the program completes, the assay plate is ready in C2 for fluorescence imaging.
Automated Duolink® Assay vs Manual Assay Results
The automated Duolink® protocol was tested using the EGFR-HER2 control kit reagents in both square and round multiwell plates. Duolink® Component Testing assays were performed testing negative (no primary EGFR/HER2 antibodies) and positive controls (both EGFR/HER2 antibodies) of EGF-treated SK-OV3 cells. Fluorescent images of manual and AAW™ workstation performance of the EGFR-HER2 control kit assay using the Duolink® Component Testing protocol show absence of EGFR-HER2 colocalization in negative controls and presence of colocalization (puncta) in positive controls (Figure 5). This result was reproducible across multiple runs and both plate geometry types (Figure 6). Semi-quantitative assessment of percent positive area staining was performed using ImageJ analysis (Figure 7).

Figure 5.Representative fluorescent images of EGFR-HER2 protein-protein interaction in EGF-treated SK-OV3 cells. The Duolink® Control Assay protocol was performed either manually or with the AAW™ workstation on EGF-treated cells. Similar EGFR-HER2 colocalization patterns (orange puncta) for negative (-/-) and positive controls (+/+) were observed between manual and automated assays. Images taken at 40x with GE In Cell Imager 2200. DAPI stain is in blue. Vertical rows include: Manual, Automated Run 1, and Automated Run 2. Horizontal rows include: -/- HER2/EGFR, +/- HER2/EGFR, -/+ HER2/EGFR, and +/+ HER2/EGFR.

Figure 6.AAW™ automation of EGFR-HER2 protein-protein interaction is compatible with both square and round multiwell plates. Similar EGFR-HER2 colocalization (red puncta) patterns for negative (-/-) and positive controls (+/+) were observed between square well and round 96-well multiwell plates. Images taken at 40x with GE In Cell Imager 2200 and 20x with BioTek® Cytation 5. DAPI stain is in blue. (Top row) square well, (Bottom row) round well. Vertical rows include: -/- HER2/EGFR, +/- HER2/EGFR, -/+ HER2/EGFR, and +/+ HER2/EGFR.

Figure 7.Assessment of percent positive staining area of EGFR-HER2 colocalization in square and round multiwell plates. A) Control EGFR-HER2 Assay on square well plate to test AAW™ workstation automated vs manual runs. Average percent positive area staining of EGFR-HER2 colocalization was determined by ImageJ analysis and confirmed positive (+/+) control resulted in higher EGFR-HER2 staining than negative control for both manual and automated assays in square well plates. B) Control EGFR-HER2 Assay on round well plate to test AAW™ workstation automated vs manual runs. Similar results were observed for round well plates as the square ones.
The AAW™ workstation automated Duolink® assay allows for both antibody testing using the Duolink® Component Testing protocol and sample screening (input up to 96) using the Duolink® Multiwell Plate protocol. Here, the Duolink® Multiwell Plate protocol was assessed on EGF-treated vs. untreated SK-OV3 cells (48 wells each). Representative fluorescent images of EGFR-HER2 colocalization for EGF-treated and untreated are shown in Figure 8a. Semi-quantitative assessment of colocalization by % positive staining area shows EGF treatment results have statistically significantly higher EGFR-HER2 interaction (~2.5x) (Figure 8b).

Figure 8.EGF treatment results in significantly greater EGFR-HER2 staining. A) Representative images of the Full Plate automated assay run on 48 samples of (Top) EGF-treated SK-OV3 cells and (Bottom) 48 samples of untreated cells. EGF treated cells displayed more robust staining than untreated cells. Images taken at 40x with GE In Cell Imager 2200. DAPI: blue and EGFR-HER2:orange. B) Quantification of percent staining area of EGFR-HER2 colocalization puncta confirms EGF treatment statistically increases protein-protein interactions over untreated cells (two-tailed T-test, p<0.003, N=48/condition).
Finally, analysis of assay times and hands-free work was compiled and compared between automated and manual assays. While cycle times are very similar, the major advantage is in the reduction of hands-on time. The Duolink® assay takes approximately 1.5 hrs on Day 1 and 5-7 hrs on Day 2 to execute. With the automated protocol 95% of that time is hands-free, and if the Temperature Module is implemented 100% is hands-free.
Summary
This article demonstrates how to utilize the Duolink® proximity ligation assay technology on the AAW™ Automated Assay Workstation and shows that the walk-away protocol will perform as well as if done manually by an experienced user.
Request more information on the AAW™ workstation below.
To continue reading please sign in or create an account.
Don't Have An Account?