MDCK Cell Culture Protocol Using a 96-well TEER Assay System
Introduction
One of the major roadblocks to the successful development of new drugs lies in understanding and testing the absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of candidate compounds. Absorption is a drug’s ability to cross epithelial and endothelial cell barriers from the point of administration to the site of action. Immortalized cell lines have been used as drug absorption models for many years and aid in the understanding of drug permeability mechanisms1.
Absorption studies can be tedious and require expertise in cell culture and assay development. Our Millicell® 96-well cell culture plate with 0.4 μm pores, polycarbonate membrane, and a tissue culture (TC)-treated surface facilitates the use of in vitro model cell lines in the measurement of drug absorption rates. Data generated using this device can ultimately provide direction to rank order the oral absorption profiles of new candidate drug compounds.
Often used in absorption studies, Madin-Darby Canine Kidney (MDCK)-II cells form “leaky” monolayers ~100 Ωxcm2. MDCK-II cells are particularly useful for permeability studies that approximate small intestine-like resistances2, but MDCK cells transfected with human MDR1 cDNA have also been used to measure passive transcellular diffusion and P-glycoprotein mediate efflux3,4,5 and CNS penetration6,7. MDCK-MDR1 monolayers typically exhibit low paracellular permeability and robust apical P-glycoprotein expression, consistent with Blood Brain Barrier (BBB)-like barrier behaviors for testing8.
The following protocol will provide guidance for the optimization of MDCK-II and MDCK-MDR1 cell growth and differentiation on the Millicell® 96-well cell culture assembly.

Figure 1.Plate diagram that depicts the Millicell® 96-well device components. A) Lid; B) 96-well cell culture plate; C) Single-well feeder tray; D) 96-well receiver plate. The plate, tray, and lid are made from clear polystyrene.
Materials
- MDCK-II cells (MTOX1300)
- MDCK-MDR1 cells (MTOX1303)
- Millicell® 96 well plate, 0.4 μm pore polycarbonate membrane, sterile (PSHT004S5)
- Millicell® ERS 3.0 Digital Voltohmmeter (MERS03000)
- Millicell® ERS 3.0 96-well Electrode (MERS0396P)
- Fetal Bovine Serum (FBS) (ES009-M)
- Minimum Essential Medium Eagle (MEM) (M2279)
- EmbryoMax MEM, Non-Essential Amino Acids (TMS-001-C)
- L-Glutamine solution (TMS-002-C)
- Penicillin & Streptomycin (P4333)
- HBSS (H8264)
- Lucifer yellow (L0144)
4-Day MDCK II & MDCK-MDR1 Growth Assay Protocol
The purpose of this experiment is to model MDCK-II and MDCK-MDR1 growth curves across 3 seeding densities. Cell growth was quantified via Trans-Epithelial Electrical Resistance (TEER) measurements using the Millicell® ERS 3.0 Digital Voltohmmeter and the 96-well electrode probe.


Figure 2. Millicell® Digital Cell Imager (DCI) 10x brightfield images of MDCK-II (left) and MDCK-MDR1 (right) cell lines.
MDCK II and MDCK-MDR1 cells were seeded into Millicell® 96-well plates at 10k, 20k, and 30k cells/well. Plates were placed back into the incubator for 24-hours to allow for cell attachment to the membrane before daily TEER measurements. The plates were left at room temperature for 20 minutes to acclimate prior to recording measurements. Since TEER is an inverse function of temperature (as temperature decreases, TEER values increase), make sure that the temperature of the plate is stable for best accuracy.

Figure 3.4-Day MDCK II & MDCK-MDR1 assay protocol; (left) 96-well plate layout from the Millicell
Millicell® Cloud Graphing
The Millicell® Cloud automatically graphs data from the Millicell® ERS 3.0 and averages data from well groupings. Through the Millicell® Cloud, researchers can create comprehensive graphs that are automatically generated, save users time, and are easily exportable (Figure 4). Researchers can pull data from different experiments by picking a device, cell line, project, and plate names to generate an automatic plot. However, when using GraphPad Prism, researchers can add more customization and statistical tests (ANOVA, T-tests).
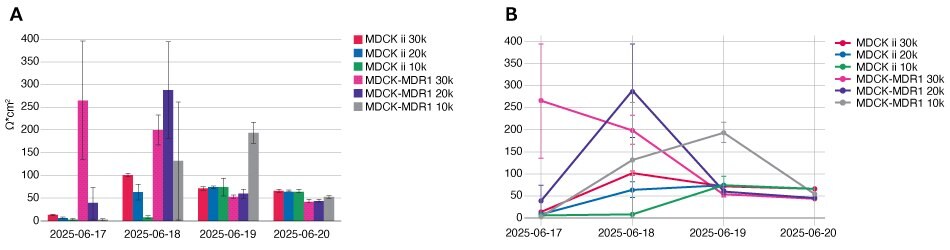

Figure 4.Millicell® Cloud and GraphPad Prism TEER Data Graphs. (top left) Millicell® Cloud bar graph; (top right) Millicell® Cloud line graph; (bottom left) GraphPad Prism bar graph; (bottom right) GraphPad Prism line graph.
Seeding Density Determination
This experiment determined that 20k cells/well is the optimal seeding density for a 4-day assay. The 30k cells/well seeding density is too high (Figure 4) and the cells already have peak resistance values after 24 hours from seeding. The 10k cells/well seeding density reached the plateau resistance values, but did not hold the plateau for 24 hours to demonstrate plateau stability. For these reasons, the 20k cells/well seeding density is optimal for this 4-day assay. In this experiment, the MDCK-II parental line has slightly higher resistance plateaued values than the MDCK-MDR1 cell line.
All three seeding densities formed a successful monolayer by 4 days post seeding as determined by lucifer yellow passthrough less than 2%. The MDCK-MDR1 cell line had slightly lower passthrough percentage than its parental MDCK-II.
Scratch Wound Assay
The scratch wound assay is a widely used in vitro method for studying cell migration and invasions through the membrane and is valuable in research areas such as cancer metastasis, immune responses, epithelial injury, and wound healing9. The assay also aids in identifying chemo attractants and evaluating the effectiveness of engineered scaffolds.

Figure 5.Scratch wound assay on membrane inserts. The epithelial layer is gently scratched with a pipet tip to remove a section of epithelial cells. After several days of culture, epithelial cells migrate into the open area.
In this assay, a confluent monolayer of epithelial cells is denuded using a pipet tip to tear off the epithelial cells. The repair is then quantified and monitored via TEER measurement. After scratching the epithelial layer, MDCK cell lines migrated over three days to close the wound, which was confirmed via lucifer yellow pass-through assay. Similar studies have been conducted to model phenotypic pulmonary fibrosis10.
Here we demonstrate how Millicell® ERS 3.0 TEER measurements correlate with scratch wounds to the cellular monolayer and how this is different from a membrane tear.
In Figure 6 (below), we demonstrate that an empty membrane insert has a baseline resistance of 29 Ω×cm2 and a LY passthrough of 43%. A successful cell monolayer had a resistance of 93 Ω×cm2 and a LY passthrough near 0%, demonstrating that the cell monolayer formed successful tight junctions.
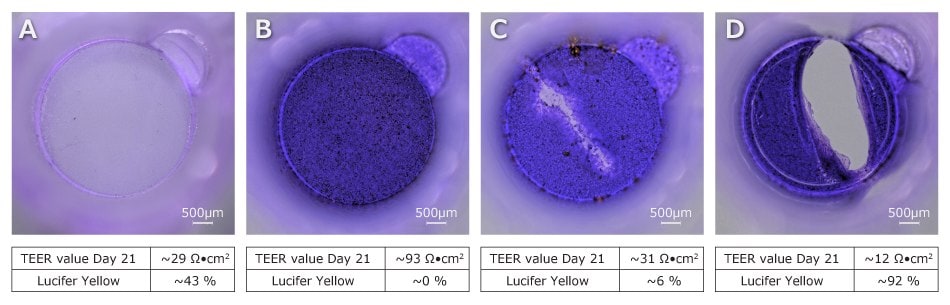
Figure 6.Representative Scratch Assay: Caco-2/TC-7 monolayers Stained with Crystal Violet. (A) A membrane with no cells; (B) full Caco-2/TC-7 monolayer after 21-days of culture; (C) monolayer that was scratched with a pipet tip during media exchange; and (D) disrupted monolayer due to puncture of the membrane with a pipet tip with associated TEER readings and Lucifer Yellow results. TEER can indicate if a monolayer or membrane has been damage prior to a permeability assay and provide assurance monolayer formation is robust.
When we introduce scratch wound to the monolayer, the TEER measurement drops to 31 Ω×cm2, which is just above the baseline TEER value, but has a 6% passthrough demonstrating that most of the monolayer is still intact. Since the signal for TEER measurement takes the path of least resistance, the signal will pass through the scratch region which resembles the cell free surface. When the membrane insert is ruptured, both the TEER value and LY passthrough drastically change. The TEER value for a broken membrane insert is ~12 Ω×cm2, which is about the resistance of media only. The LY% passthrough is 92% in this sample because there is basically nothing preventing the mixing of apical and basolateral chamber fluids.
These examples exemplify the importance of having proper controls. Having a blank cell free control will alert to potential damage to your membrane inserts if the sample well has a lower resistance than your blank well. Similarly, if the sample well suddenly measures resistance similar to background, it could indicate that something poked or scratch the cells on the membrane.
Scratch Wound Assay with MDCK-II and MDCK-MDR1 Cells
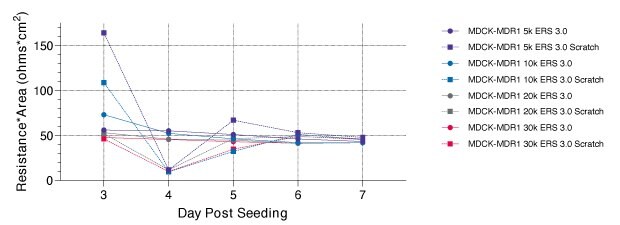
In this experiment, MDCK-II and MDCK-MDR1 cells were seeded onto Millicell® 96-well membrane insert plates at seeding densities 30k, 20k, 10k, and 5k cells per well. Cells were grown for three days before daily TEER measurements. On day four, the cell membranes were scratched with a 20 μL pipet tip from the 12 o’clock position straight down to the 6 o’clock position, just prior to measuring teer with Millicell® ERS 3.0.
All scratched wells regardless of cell line exhibited the same TEER value ~11-16 Ω×cm2. Notably, the MDCK-MDR1 cell line seemed to recover from scratch wound almost fully by 24 hours post scratch, but MDCK-II cells took nearly double the recovery time to reach the same resistance values (Figure 6). Once cultures reached day seven, day three from scratch, a lucifer yellow assay was performed to check for barrier healing. All groups had <1% passthrough and showed successful repair from scratch assay.
MDCK-MDR1 7-Day ERS 3.0
MDCK-II 7-Day ERS 3.0

Figure 7.Millicell® ERS 3.0 data. For MDCK-II (bottom) and MDCK-MDR1 (top) cell lines, the seven-day experiment was run with scratch inflicted on day four just prior to measuring resistance. In this experiment, both cell lines were seeded at variable seeding densities 5k, 10k, 20k, and 30k cells per well.
Conclusion
MDCK-II cells are a viable alternative to Caco-2 cells as an in vitro model system to evaluate potential drug candidates. The drug transport rates measured using this model system can help determine the probability of the drug being absorbed orally. As shown above, the Millicell® 96-well plate is ideal for culturing MDCK-II and MDCK-MDR1 cells and performing these analyses.
The optimal seeding density for MDCK cell lines was determined for 4-day and 7-day cultures in this protocol note. Figure 2 illustrates the template used for cell dilutions. After 4 days in culture, the resistance (TEER) and lucifer yellow (LY) passage were determined as described. The resistance of MDCK-II cell monolayers at the densities chosen was ~65-72 Ω×cm2 and ~44-54 Ω×cm2 for MDCK-MDR1 cells; resistance values returned to healthy monolayer values after the scratch wound assay was performed.
Millicell® ERS 3.0 Tips and Tricks
Take into consideration that resistance (TEER) values vary based on several factors; best practice is to control for as many of these factors as possible:
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?