Hayashi Catalysts
Efficient and Selective Catalysts for Asymmetric Synthesis
[RhOH(S)-BINAP]2 (1) and Rh(I) Pre-catalysts
The Hayashi group at Kyoto University has spearheaded the development of rhodium-based catalysts applied in the asymmetric, conjugate addition of arylboronic acids to C=C bonds.1 They utilized Rh-BINAP catalysts to effect several advantages over other enantioselective 1,4-addition reactions: 1) high selectivities (> 95% ee) have been attained, 2) the reaction is performed in an aqueous environment, 3) the reaction temperature is > rt (60 - 90 °C) and thus is advantageous for process design, 4) a multitude of aryl and alkenyl groups can be incorporated, and 5) a variety of electron-deficient olefins can be effectively coupled with boronic acids in an asymmetric fashion.
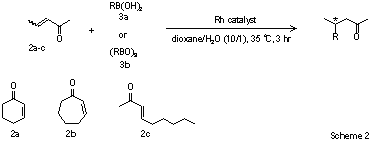
Sigma-Aldrich is now pleased to offer the latest technology from Hayashi, including both the pre-catalysts suitable for facile conversion into the active species as well as the catalysts themselves (Scheme 1). Rh-dimer catalyst 1 offers additional benefits over its monomeric counterparts: 1) the reaction is carried out at ambient temperatures, which limits the amount of boron reagent needed as a result of a disfavoring of boron decomposition at higher temperatures, 2) the yield of 1,4-addition product is typically higher due to a retarding of arylboronic acid hydrolysis, and 3) the enantioselectivities are always higher in reactions catalyzed by [RhOH((S)-BINAP]2 than by [Rh(acac)(BINAP)]2. In general the Hayashi system displays impressive levels of enantiocontrol in the reactions of both acyclic and cyclic enones of varying electronic character affording highly enantioenriched products in excellent yields (Scheme 2, Table 1).
These Rh(I) catalysts have also been applied in the enantioselective synthesis of 2-aryl-4-piperidones (Scheme 3), which are known to be building blocks for biologically active molecules such as the tachykinin antagonists.3,4 It should be noted that the asymmetric, catalytic 1,4-addition of organoboron or organozinc reagents involves a broad scope of electronically and sterically diverse reagents. In all cases the authors have convincingly produced highly enantiopure 2-aryl-4-piperidones in good to excellent yields.

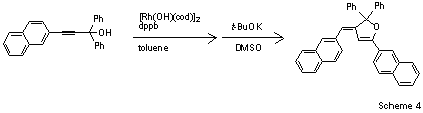
Miura and co-workers have explored the use of [Rh(OH)(cod)]2 in the regio- and stereoselective homocoupling of γ-arylated tert-propargyl alcohols (Scheme 4).5 This efficient and unprecedented alkyne coupling catalyzed by a Rh(I)-dppp complex (dppb = diphenylphosphinobutane) liberates ketone byproduct through β-carbon elimination to afford 2-hydroxymethyl-(E)-enynes. These organic compounds are useful precursors to dihydrofuran derivatives that produce intense fluorescent emissions in the solid state.
To continue reading please sign in or create an account.
Don't Have An Account?


