Metal Borohydrides as Hydrogen Storage Materials
Introduction
To meet current Department of Energy (DOE) targets, the materials for hydrogen storage should possess high hydrogen content, low heats of dehydrogenation (to minimize the energy penalty), and fast kinetics of hydrogen desorption at operation temperatures of a PEM fuel cell (80–120 °C). Of the number of different options being considered, solid metal hydrides that reversibly desorb large amounts of hydrogen are extremely attractive as base materials for hydrogen storage due to process simplicity, low operation pressures, and relatively low cost.
Early work in the area of hydrogen storage concentrated on intermetallic hydrides like LaNi5 and TiFe, which showed very good sorption/desorption kinetics but had low hydrogen storage capacities (below 2% of hydrogen by weight). Numerous attempts to increase their capacity and keep good kinetics by making polymetallic compositions with lightweight metals were unsuccessful.
A subsequent wave of research interest was aimed at sodium aluminum hydride (or alumohydride, or alanate), NaAlH4, after Bogdanovicˇ found that titanium catalysts decrease the desorption temperature of NaAlH4 and make this process reversible.1 This work showed that complex metal hydrides might be used as reversible hydrogen storage materials. However, relatively low capacities of complex metal alumohydrides, except LiAlH4 (10.5%), and complexity of their decomposition constitute a serious problem to be resolved before their commercialization. Aluminum hydride AlH3, which contains 10 wt. % of hydrogen, smoothly decomposes in one step at moderate temperature but can be regenerated only at very high pressure (24 Kbar).2
Complex metal borohydrides M(BH4)n with their high hydrogen content (Table 1) and the potential to meet DOE targets are of growing interest. Their synthesis and properties related to hydrogen storage are briefly discussed below.
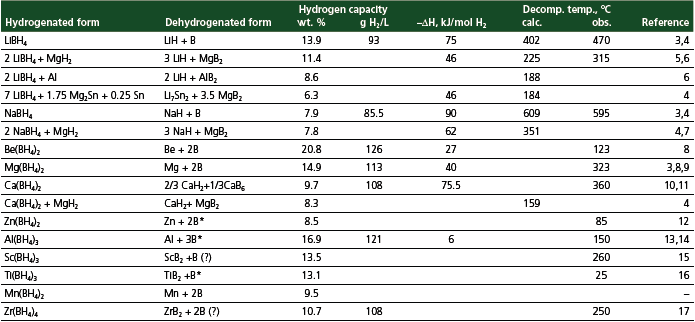
Table 1.Properties of metal borohydrides.
General Synthetic Methods
The first metal borohydride, LiBH4, was synthesized by Schlesinger and Brown more than 65 years ago using the reaction of ethyllithium with diborane.18 Although discovered at about the same time, the synthesis of sodium borohydride, NaBH4, was reported much later.19 NaBH4 is the most widely commercially produced borohydride and is used in the paper and textile industries, and as a reducing agent in organic synthesis.20 It is also a commonly used starting material for synthesis of other metal borohydrides.
The direct synthesis from the elements at elevated pressure and temperature was claimed for borohydrides of Li, Na, K, Mg, and Ba.21 In practice only indirect methods are used for synthesis of metal borohydrides.
Four general methods are used for the preparation of metal borohydrides: i) addition of diborane, B2H6, to metal hydrides, ii) reaction of B2H6 with metal alkyls or metal alkoxides, iii) reaction of metal hydrides with boron compounds, and iv) exchange reaction between metal borohydrides and other metals salts, mostly halides. Historically, reactions based on the use of diborane were employed first. If the metal hydride is stable, such as hydrides of Li, Na, and Mg, reaction (Eq. 1) proceeds smoothly to the metal borohydride. However, if the metal hydride is unstable, like hydrides of Be and Al, a metal alkyl or alkoxide should be used (Eq. 2).
MHn + n/2 B2H6 ------> M(BH4)n (1)
Al2Me6 + 4 B2H6 ------> 2 Al(BH4)3 + 2 BMe3 (2)
In the case of alkali earth metal borohydrides (M = Mg, Ca, Sr, Ba) alkyldiboranes are used instead of B2H6, the reactions are carried out without solvent (Eq. 3), and boralkyls are removed by distillation to give pure products.22
MH2 + 3 B2H2Pr4 ------> M(BH4)2 + 4 BPr3 (3)
The toxicity, high flammability, and low thermal stability of diborane make processes based on Eqs. 1 and 2 impractical. Instead, processes using in situ generation of diborane have been developed. Under the right conditions, reaction of metal hydrides (Eq. 4) or alumohydrides (Eq. 5) with BF3 etherate or alkylborates leads to the preparation of metal borohydrides via intermediate B2H6.
4 LiH + BF3·Et2O ------> LiBH4 + 3 LiF + Et2O (4)
LiAlH4 + B(OMe)3 ------> LiBH4 + Al(OMe)3 (5)
The exchange reaction (Eq. 6) is a common method for the preparation of binary metal borohydrides M(BH4)n as well as multiple metal borohydride complexes stabilized by donor ligands.
MXn + n M’BH4 ------> M(BH4)n + n M’X (6)
Li or Na borohydrides are usually used as a source of BH4 - group but borohydrides of other metals (K, Ca, Al) can also be used. This reaction usually takes place in donor solvents (ethers, amines), where one or both reagents are soluble but one of the reaction products, usually an alkali metal chloride, is not. Metal borohydrides are isolated from solution as solvates with one or more molecules of the solvent. Therefore, this process requires an additional desolvation step. However, thermal desolvation, often in vacuum, in some cases can lead to the decomposition of M(BH4)n with H2 evolution preceding the desolvation point. In such cases, unsolvated borohydrides can be prepared by mechanochemical exchange reactions (e.g. using ballmilling); see also the article by V. Balema in this issue. This method is very convenient for volatile borohydrides, such as those of Be, Al, and Zr, which can be isolated by distillation or sublimation.23 However, for nonvolatile M(BH4)n the removal of the M’X byproduct is very difficult.
Synthesis and Properties of Some Metal Borohydrides
Lithium borohydride LiBH4 (Product No. 222356) has been prepared using all of the previously described synthetic methods. Probably, the most practical one is the exchange reaction of LiCl with NaBH4 in isopropylamine.
LiBH4 is a white solid with density of 0.68 g/cm3. It exists in two crystal modifications (orthorhombic transforms to tetragonal at 108 °C) and melts at 278 °C. It reacts violently with water to produce H2, but is relatively stable in dry air.
Decomposition of liquid LiBH4 with hydrogen evolution starts at 320–380 °C (470 °C under 10 atm H2 pressure).3 In earlier work it had been reported that during the decomposition only 50% of hydrogen was released and an unidentified phase “LiBH2” formed.18 In later work it was reported that the LiBH4 decomposition liberates ~80% of total hydrogen at ambient pressure24 and 75% at 10 atm H2 (Eq. 7).3 However, in the most recent publication25 the formation of “LiBH2” and perhaps other intermediates (at low heating rates) has been confirmed.
LiBH4 ------> LiH + B + 3/2 H2 (7)
Sodium borohydride NaBH4 (Product No. 480886; 452882; 452874; 452173; 452165) is a high melting (505 °C) solid with relatively low reactivity. Its basic solution in water is stable to hydrolysis.
Two processes for NaBH4 manufacturing have been commercialized. Probably the most convenient is the borate method, developed in the U.S., where methylborate reacts with sodium hydride in mineral oil (Eq. 8). This reaction does not require hydrogen pressure but occurs at 250–280 °C, temperatures necessary to melt and disproportionate the intermediate Na[HB(OCH3)3]. Dissolution of the reaction mixture in water and extraction with isopropylamine gives the dihydrate that can be desolvated by heating in vacuum to give pure NaBH4.
4 NaH + B(OCH3)3 ------> NaBH4 + 3 NaOCH3 (8)
The borosilicate process developed by Bayer (Eq. 9) uses less expensive boron compounds but requires higher temperature (400–500 °C) and hydrogen pressure. Isolation of NaBH4 involves extraction with liquid NH3 under pressure.
(Na2B4O7 + 7 SiO2) + 16 Na + 8 H2 -------> 4 NaBH4 + 7 Na2SiO3 (9)
Because of the potential use of NaBH4 for hydrogen generation by catalytic hydrolysis, its regeneration from the borate NaBO2 is being studied intensively. An analysis of the thermodynamics of more than 30 reactions leading to NaBH4 showed that the use of calcium hydride (Eq. 10) for this purpose is the most favorable approach.26
NaBO2 + 2 CaH2 ------> NaBH4 + 2 CaO (10)
Beryllium borohydride Be(BH4)2 has the highest total hydrogen capacity (Table 1). It was originally prepared by reaction of BeMe2 with diborane27 and, more conveniently, by mechanochemical exchange reaction of BeCl2 with alkali metal borohydrides followed by vacuum distillation at 140 °C.28 This covalent compound is built of helical polymeric chains –BeH2BH2BeH2BH2– and terminal bidentate BH4-groups.29 The covalent character of Be–BH4 bonding explains the high volatility of Be(BH4)2 and, therefore, also its extremely high reactivity (it explodes on contact with air and moisture). Unfortunately, the extreme toxicity of beryllium, and very high reactivity of Be(BH4)2, makes this material unsuitable for hydrogen storage despite low decomposition temperature and ∆Hf.
Magnesium borohydride Mg(BH4)2 was first reported more than 50 years ago.30 Plešek and Herˇmánek isolated the unsolvated magnesium borohydride using reaction of MgH2 with diborane (Eq. 1).31 Konoplev and Bakulina reported the synthesis of unsolvated Mg(BH4)2 by exchange reaction (Eq. 6) of MgCl2 with NaBH4 and provided the X-ray diffraction (XRD) pattern of its two crystal modifications.32 Literature data on solvent-free Mg(BH4)2 synthesis and properties are contradictory as well as attempts to predict its structure. Our group at GE GRC prepared two crystal modifications of Mg(BH4)2 with an XRD pattern different from the reported in Ref. 32 using a modified exchange method. A hexagonal phase, which is stable at room temperature, converts to an orthorhombic phase at 185 °C, which also can be stored at room temperature.33 Both phases have complex networks of corner-sharing tetrahedra consisting of a central Mg atom and four BH4 units. Differential scanning calorimetry (DSC) of Mg(BH4)2 shows two endothermic peaks at 300 and 376 °C, and one exothermic peak at 357 °C that can be assigned to decomposition of Mg(BH4)2, crystallization of amorphous MgH2, and decomposition of MgH2 into elements (Eq. 11).33 Structures of both phases of Mg(BH4)2 and their properties will be published elsewhere.
Mg(BH4)2 ----> 3 H2 + 2 B + MgH2 (amorph) ----> MgH2 (cryst) ----> Mg + H2 (11)
Calcium borohydride Ca(BH4)2 (Product No. 389986; 21057) has been prepared by the reaction of diborane with calcium hydride34 or alkoxides35 but an exchange reaction between CaCl2 and NaBH4 in a ball mill10 or in THF36 are more convenient ways of making it. Desolvation of the Ca(BH4)2·2THF adduct occurs easily in vacuum at 190 °C.36 Ca(BH4)2 is a nonvolatile solid with density 1.12 g/cm3. It is completely stable in dry air and soluble in water without decomposition.
Unsolvated Ca(BH4)2 has an ionic structure with Ca2+ ions surrounded by six tetrahedral BH4-groups and each BH4-group contacts with three Ca2+ ions.11 Despite relatively low hydrogen capacity, the volumetric hydrogen density of Ca(BH4)2 is comparable with that of Mg(BH4)2 due to its higher density (Table 1). Decomposition of Ca(BH4)2 begins at 360 °C but is complete only at 500 °C and releases 9.6% hydrogen according to Eq. 12, which was predicted on the basis of the calculated heat of formation.11
3 Ca(BH4)2 ------> 2 CaH2 + CaB6 + 10 H2 (12)
Zinc borohydride Zn(BH4)2 in a solvent-free form was made by ball-milling of ZnCl2 and NaBH4 as a mixture with NaCl.12 According to DSC data, unsolvated Zn(BH4)2 melts at about 95 °C with decomposition.12 The decomposition is endothermic and proceeds with the formation of diborane (Eq. 13).12
Zn(BH4)2 ------> Zn + B2H6 + H2 (13)
Aluminum borohydride Al(BH4)3 was first prepared by reaction of trimethylaluminum with diborane,18 but the mechanochemical exchange reaction of AlCl3 and NaBH4 followed by vacuum distillation of the target product into cooled traps is much more practical.37 Al(BH4)3 is a liquid (m.p. –64 °C) and its structure was determined by low temperature single crystal X-ray diffraction.38 Both modifications (phase transformation temperature is about 180 K) are built of discrete molecular units with similar geometry: the aluminum atom surrounded by three bidentate BH4-groups with AlH2B planes perpendicular to the AlB3 plane.
Decomposition of Al(BH4)3 starts at 150 °C and has a first order kinetics that is not affected by the presence of hydrogen.14 Calculated heat of formation is estimated as –5.5 kJ/mol H2 with the zero-point energy correction.13
Scandium borohydride Sc(BH4)3. Surprisingly little is known about scandium borohydride Sc(BH4)3. Solvates Sc(BH4)3·2THF and Sc(BH4)3·DME are known but these complexes can not be desolvated. Ball-milling of ScCl3 and LiBH4 yields an amorphous product with n(B–H) vibration in the range 2200–2500 cm–1 in the Raman spectrum.15 The decomposition of this product starts above 150 °C and has a maximum at ~260 °C.15
Titanium borohydride Ti(BH4)3 was prepared by the reaction of LiBH4 with TiCl439 or TiCl340 (titanium fluoride salts do not react) and isolated by low temperature vacuum sublimation. Ti(BH4)3 is a white volatile solid. Electron diffraction in the gas phase showed a monomer molecule with tridentate BH4-groups. Based on its physical properties, the bonding in titanium borohydride crystals should be similar to that in molecular crystals of Al(BH4)3. Ti(BH4)3 is thermally unstable and decomposes to TiB2, H2, and B2H6 at 20 °C.16
Manganese borohydride Mn(BH4)2 was isolated only as a solvate with ethers or amines. Attempts to desolvate Mn(BH4)2·nL resulted in its decomposition with the ligand’s destruction.41
Zirconium borohydride Zr(BH4)4 is conveniently prepared by mechanochemical synthesis using NaZrF5 and ZrCl4 in combination with Al(BH4)3 and alkali metal borohydrides.39 Zr(BH4)4 decomposition produces a solid with the composition ZrB2.76–3.74.17 The only crystalline phase in this product is ZrB2, therefore the rest is probably amorphous boron. The difference in the B:Zr ratio in starting material and products points to the possible evolution of diborane during pyrolysis.
Thermodynamic Properties of Metal Borohydrides
The heat of dehydrogenation (or reverse reaction of hydrogenation) is a very important parameter of hydrogen storage materials. It is desirable that this value is about 40 kJ/mol H2 to provide a reasonable part of lower heating value (LHV), and a reasonable equilibrium hydrogen pressure at 80–120 °C. Despite the long history of metal borohydrides, only a few data on their thermodynamic properties has been published in the open literature.8 Direct calorimetric measurements of very reactive and volatile compounds are very challenging. Therefore, calculation of thermodynamic characteristics of metal borohydrides using density functional theory (DFT) became a common practice. These calculations have a good correlation with experimental data only if the crystal structure of compounds is known (including coordinates of hydrogen atoms). It should be noted that standard enthalpy of formation (reverse of reaction 14) and enthalpy of dehydrogenation (∆Hdes) may not have the same absolute value because dehydrogenation of M(BH4)n may occur with the formation of the corresponding metal hydride or/and boride (compare Eqs. 7, 12 and 14). Known data on enthalpies of decomposition of M(BH4)n are given in Table 1. It was proposed that there is a linear relationship between heat of formation of M(BH4)n and electronegativity of the metal.15 There is also an almost linear correlation between desorption temperature and estimated ∆Hdes.15
M(BH4)n ------> M + nB + 2n H2 (14)
A very promising concept of destabilization of metal borohydrides using the formation of metal borides was suggested by Vajo et al.5 The concept is based on the use of stable borides (MgB2,5 AlB2 6), possibly in combination with lithium alloys (Li7Sn2, Li0.3Mg0.7)4,42 to effectively decrease ∆Hdes (Eqs. 15–17). Formation of borides instead at elemental boron decreases reaction enthalpies by 10–25 kJ/mol H2, which in turn is related to the decrease of the decomposition temperature by 150–250 °C (Table 1). To further lower the decomposition temperature, it was proposed to use substitution of Li+ for cations such as Mg2+.43 This idea worked well for LiNH2, which has the structure similar to Mg(NH2)2,44 but in the case where crystal structures are different, it may not work.
2 LiBH4 + MgH2 -------> 2 LiH + MgB2 + 4 H2 (15)
2 LiBH4 + Al -------> 2 LiH + AlB2 + 3 H2 (16)
7 LiBH4 + 1.75 Mg2Sn + 0.25 Sn ------> 2 Li7Sn2 + 3.5 MgB2 + 14 H2 (17)
Metal Borohydrides as Hydrogen Storage Materials
The following major challenges in the chemistry of M(BH4)n must be solved before their commercial use as hydrogen storage materials: high temperature of dehydrogenation, lack of reversibility of the dehydrogenation reaction, slow kinetics of dehydrogenation and hydrogenation, evolution of diborane during dehydrogenation, and, finally, high cost of borohydrides. Taking into account these considerations and hydrogen content, the most promising materials for hydrogen storage are borohydrides of lithium, magnesium, and calcium and their compositions. At first sight, Ca(BH4)2 has low gravimetric hydrogen storage capacity, but its volumetric capacity is higher than that of LiBH4 and comparable with Mg(BH4)2 .
It was found that mixing LiBH4 with silica powder (3:1) substantially decreased its decomposition temperature.25 In this case hydrogen release starts at 200 °C and occurs in two steps with a maximum at 320 °C (broad desorption peak) and 453 °C (sharp peak). The mechanism of such catalytic action is presently unclear. As mentioned above, destabilization of M(BH4)n due to boride formation substantially decreases decomposition position temperature. However, the observed temperature decrease is noticeably lower than that predicted by thermodynamics (Table 1). Probably, the kinetics of metal boride formation is slow and dehydrogenation occurs by parallel pathways with formation of both metal boride and elemental boron.
Borohydride formation by reaction of hydrogen with boron and hydrides of Li, Na, Mg, and Ba at 600–700 °C was described in patent literature.21 Reaction with LiH requires 150 bar H2 while in the case of MgH2 a much higher pressure (800 bar) is needed. The use of MgB2 (instead of B) with LiH decreases the hydrogenation pressure and temperature to 100 bar and 300 °C, respectively.5 Hydrogenation of the mixtures of MgB2 with hydrides of sodium and calcium allowed synthesis of NaBH4 and Ca(BH4)2 at 200 bar and 300 °C.4 Reaction time is rather long (24 h) even at 350 bar and 400 °C.
It should be noted that effective catalysts of dehydrogenation/ hydrogenation of metal borohydrides, like titanium catalysts for NaAlH4,1 have not been found yet (except silica for LiBH4).25 Further efforts to make the decomposition of Mg(BH4)2 reversible as well as improving the reaction kinetics of Li and Ca borohydrides should concentrate on the development of catalysts for these reactions.
Materials
REFERENCES
To continue reading please sign in or create an account.
Don't Have An Account?